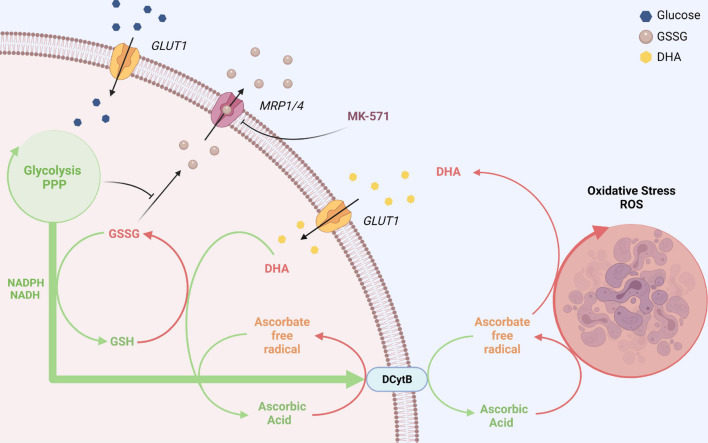
FIGURE 7.
GLUT1 and DCytb-mediated vitamin C recycling as a common feature of erythrocytes from vitamin C auxotroph mammals? The plasma membrane of human erythrocytes contains high amounts of GLUT1 and DCytb. The GLUT1 glucose transporter not only facilitates the uptake of glucose but also that of DHA, the oxidized form of vitamin C. Fast intracellular reduction of DHA to AA is ensured by high cellular GSH concentrations and the high abundance of the DHA reductase GSTO-1. Thus, spontaneous decomposition and irreversible loss of the labile DHA is prevented. DHA-dependent generation of GSSG and its export via MRP1/4 is prevented in the presence of physiological glucose concentrations. Furthermore, increased intracellular AA levels reduce intracellular ROS levels (not depicted here) and fuel DCytb-mediated plasma membrane electron transport (PMET). While in duodenal enterocytes DCytb mediates Fe3+ reduction for dietary iron absorption, the main extracellular substrate of DCytb in the erythrocyte membrane appears to be ascorbate free radical (AFR), which is consequently reduced to AA. Both processes, (i) uptake of DHA and its intracellular reduction, and (ii) DCytb-mediated PMET activity which recycles extracellular AA from AFR (generated upon reduction of ROS in the plasma), can be considered a “vitamin C recycling mechanism.” This underestimated physiological function of human erythrocytes likely contributes to (i) oxidative stress defense in blood and (ii) sustainability of systemic AA supply, considering the fluctuating dietary uptake. The importance of this system is underlined by the finding that, in contrast to most other mammalian species, erythrocytes of vitamin C auxotroph mammals harbor GLUT1 and possibly also DCytb (see section “Discussion”). “Created with BioRender.com”.