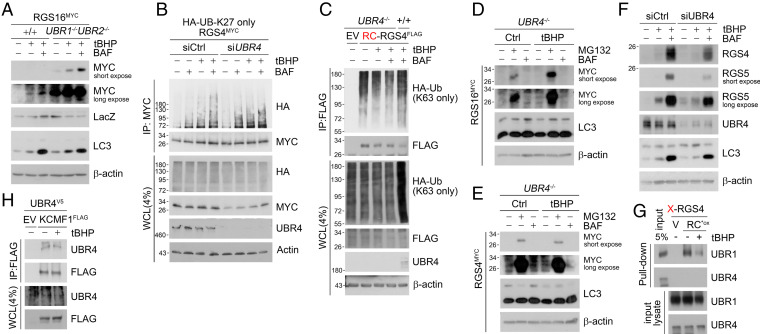
Fig. 5.
UBR4 is responsible for K27-linked polyubiquitin chain ligation. (A) WB of RGS16MYC transfected in WT and UBR1−/−UBR2−/− MEF cells with tBHP (250 μΜ, 6 h), BAF (200 nM, 6 h) treatment. (B) Denaturation IP of HEK293T cells transfected with HA-Ub-K27 only mutant and RGS4MYC and treated with tBHP (250 μΜ, 6 h), BAF (200 nM, 6 h), or both under UBR4 interference (48 h). (C) Denaturation IP in WT and UBR4−/− MEF cells expressing RCRGS4FLAG and HA-Ub-K63 only mutant and treated with tBHP, BAF, or both (same as A). (D and E) WB of RGS4MYC and RGS16MYC overexpressed in UBR4−/− MEF cells with tBHP (250 μM, 6 h), MG132 (10 μM, 6 h), and BAF (200 nM, 6 h) treatment. (F) SH-SY5Y cells with UBR4 interference (48 h) and tBHP (250 μΜ, 6 h), BAF (200 nM, 6 h) treatment (G) In vitro peptide pulldown assay of X-RGS4 (X = V, or RCO3) 12-mer peptide using endogenous HEK293T cell lysates added with tBHP (250 μΜ). (H) Co-IP of HEK293T cells expressing UBR4V5 and KCMF1FLAG treated with tBHP (250 μΜ, 6 h).