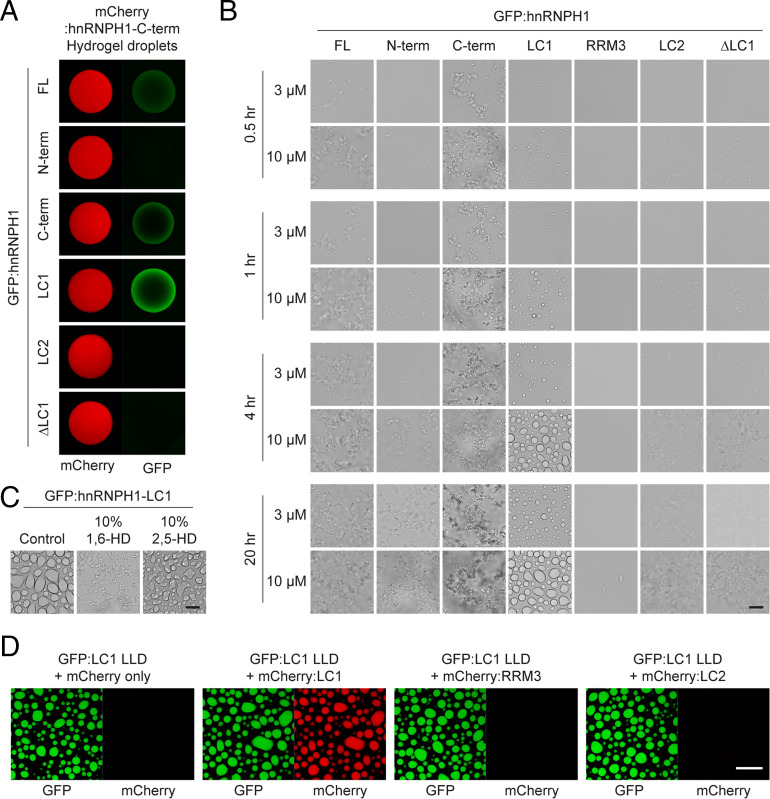
Fig. 2.
Hydrogel binding and phase separation of the LC1 domain of hnRNPH1. (A) Hydrogel droplets composed of mCherry-linked C-terminal half of hnRNPH1 were challenged with soluble proteins of GFP-linked full-length (FL), N-terminal half (N-term), C-terminal half (C-term), LC1 domain, LC2 domain, or ΔLC1 hnRNPH1. Upon overnight incubation, trapping of the GFP-linked proteins by the mCherry hydrogel droplets was detected using confocal microscopy. (B) Phase separation of GFP-linked different regions of hnRNPH1 proteins into LLDs. Different concentrations (3 or 10 µM) of GFP-linked recombinant proteins were incubated in the presence of 10% PEG for indicated time periods. The LLDs were visualized using light microscopy. (Scale bar, 10 µm.) (C) Melting of LLDs formed from the hnRNPH1 LC1 domain by 1,6- or 2,5-HDs. The droplets were visualized using light microscopy. (Scale bar, 10 µm.) (D) Incorporation of 1 µM of mCherry alone, mCherry-linked LC1, RRM3, or LC2 domains of hnRNPH1 into LLDs composed of 10 µM GFP-linked LC1 domain of hnRNPH1. Upon overnight incubation, the mCherry signals incorporated to the GFP LLDs were assessed using confocal microscopy. (Scale bar, 20 µm.)