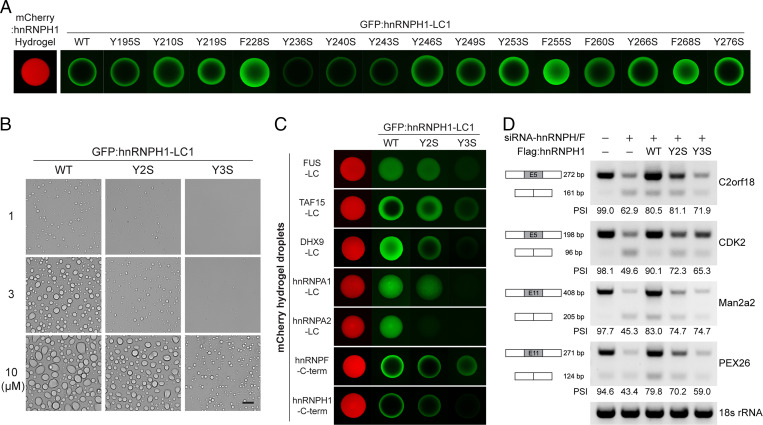
Fig. 4.
Effects of Y-to-S mutations on phase separation and function of hnRNPH1. (A) The recombinant proteins of the WT or Y/F-to-S mutant hnRNPH1 LC1 domains with N-terminal GFP tag were incubated with hydrogel droplets composed of the mCherry-linked hnRNPH1 C-terminal half. Upon overnight incubation, hydrogel-trapping of the GFP-linked LC1 domains was analyzed using confocal microscopy. (B) For LLD formation assay, 1, 3, or 10 µM of the purified recombinant proteins of GFP-linked WT, Y210/219S (Y2S), and Y236/240/243S (Y3S) were incubated in buffer containing 150 mM NaCl and 10% level of the crowding agent, PEG. The LLDs were visualized by light microscopy. (Scale bar, 10 µm.) (C) GFP-linked proteins of WT, Y2S, or Y3S LC1 domain were incubated with different kinds of mCherry hydrogel droplets. Hydrogel binding of the GFP-linked proteins was analyzed by confocal microscopy. (D) RT-PCR was performed to analyze the splicing of the exon 5 of C2orf18, exon 5 of CDK2, exon 11 of Man2a2, and exon 11 of PEX26 transcripts. The mean value of PSI levels obtained from three independent experiments is shown.