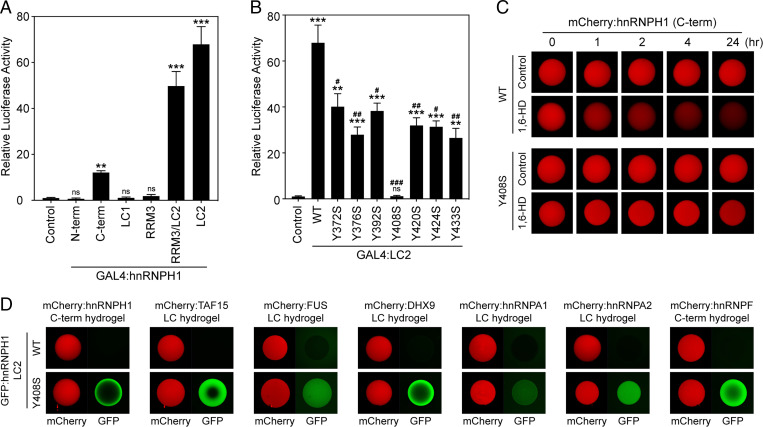
Fig. 5.
The LC2 domain of hnRNPH1 is required for transcriptional activity as a fusion protein with DBD. (A) The indicated regions of hnRNPH1 were linked to the DBD of GAL4 and assayed for activation of GAL4-dependent firefly luciferase reporter gene in HEK-293T cells. Relative luciferase activity compared to GAL4-DBD-only control. (B) Transcriptional activity of the WT or Y-to-S mutant LC2 domains of hnRNPH1 linked to GAL4-DBD were analyzed in HEK-293T cells. (A and B) One-way ANOVA was used to evaluate statistical significance. Mean ± SEM from three independent assays; ns, not significant versus control; *P < 0.01, **P < 0.001, and ***P < 0.0001 versus control; and #P < 0.01, ##P < 0.001, and ###P < 0.0001 versus WT. (C) Melting of mCherry hydrogel droplets composed of WT or Y408S C-terminal half of hnRNPH1 15% levels of an aliphatic alcohol, 1,6-HD. The hydrogel droplets were imaged by confocal microscopy at indicated time points after addition of 1,6-HD. (D) Different kinds of mCherry hydrogel droplets were challenged with GFP-linked proteins of WT or Y408S LC2 domains of hnRNPH1. Trapping of the GFP-linked proteins by the hydrogel droplets was analyzed by confocal microscopy.