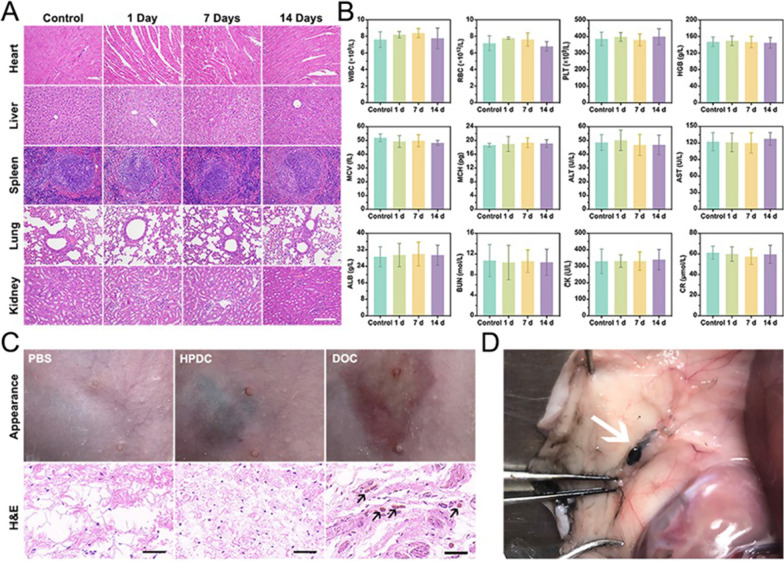
Fig. 7.
In vivo biosafety evaluation and anatomical location of HPDC NPs. A H&E stained images acquired from the major organs (heart, liver, spleen, lung, and kidney) of female rats after the subcutaneous injection with PBS (control) and HPDC NPs (1, 7, and 14 days) (Scale bars: 200 μm). B Routine blood parameters and biochemical indexes examination of the female rats after subcutaneous injection with PBS (control) and HPDC NPs for 1, 7, and 14 days feeding, including white blood cell (WBC), red blood cell (RBC), platelet (PLT), hemoglobin (HGB), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), alanine aminotransferase (ALT), aspartate aminotransferase (AST), albumin (ALB), blood urea nitrogen (BUN), creatine kinase (CK), and creatinine (CR) (n = 3 per group). C Appearance and H&E stained images of the skin 2 h after the subcutaneous injection of PBS, HPDC NPs and DOC (Scale bars: 50 μm). D Anatomical location of lymph nodes after the subcutaneous injection of HPDC NPs