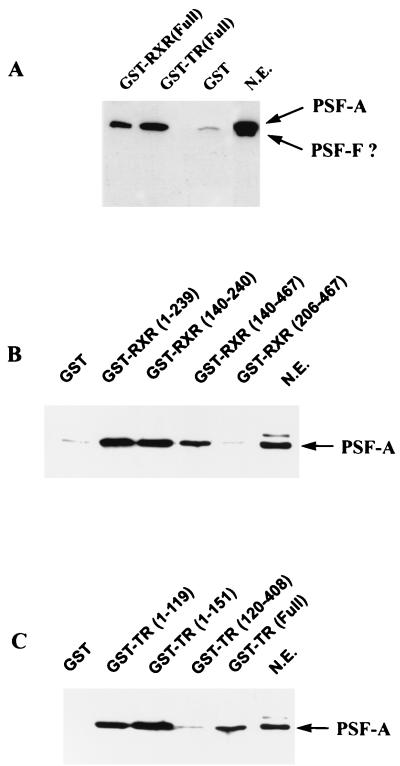
FIG. 5.
RXR and TR selectively bind PSF-A. (A) 293T cells were transfected with the mammalian GST expression vector pEBG alone or pEBG expressing full-length TR or RXR. Nuclear extracts expressing equivalent amounts of GST, GST-RXR, or GST-TR were incubated with glutathione-agarose beads. The bound proteins were then analyzed by Western blotting with anti-PSF antibody. In this experiment, the gel was electrophoresed for a longer time than normal. Under these conditions, PSF electrophoreses as a broad, partially resolved doublet, although a very short autoradiographic exposure shows two distinct bands. The size of these bands are consistent with the proteins being PSF-A and PSF-F. Since we cannot precisely determine whether the lower-molecular-weight species is PSF-F or a degradation product of PSF-A, we have indicated the band with a ?. Although both forms are present in the nuclear extract (N.E.), only PSF-A binds to the receptors. (B and C) PSF-A binding to RXR and TR involves the DBDs of the receptors. An equal amount of HeLa nuclear extract (500 μg) was incubated with equimolar amounts of bacterial expressed GST fusion proteins containing different regions of RXR or TR as indicated. The glutathione-agarose beads were washed, and the bound proteins were analyzed by Western blotting with anti-PSF antibody. PSF-A binds only to the GST-receptor fusion proteins which contain the DBD of RXR or TR. The LBDs of RXR(206–467) and TR(120–408) do not bind PSF-A.