Abstract
Background & Aims
We aimed to assess the safety and immunogenicity of inactivated whole-virion severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccines in patients with chronic liver diseases (CLD) in this study.
Methods
This was a prospective, multi-center, open-label study. Participants aged over 18 years with confirmed CLD and healthy volunteers were enrolled. All participants received 2 doses of inactivated whole-virion SARS-CoV-2 vaccines. Adverse reactions were recorded within 14 days after any dose of SARS-CoV-2 vaccine, laboratory testing results were collected after the second dose, and serum samples of enrolled subjects were collected and tested for SARS-CoV-2 neutralizing antibodies at least 14 days after the second dose.
Results
A total of 581 participants (437 patients with CLD and 144 healthy volunteers) were enrolled from 15 sites in China. Most adverse reactions were mild and transient, and injection site pain (n = 36; 8.2%) was the most frequently reported adverse event. Three participants had grade 3 aminopherase elevation (defined as alanine aminopherase >5 upper limits of normal) after the second dose of inactivated whole-virion SARS-CoV-2 vaccination, and only 1 of them was judged as severe adverse event potentially related to SARS-CoV-2 vaccination. The positive rates of SARS-CoV-2 neutralizing antibodies were 76.8% in the noncirrhotic CLD group, 78.9% in the compensated cirrhotic group, 76.7% in the decompensated cirrhotic group (P = .894 among CLD subgroups), and 90.3% in healthy controls (P = .008 vs CLD group).
Conclusion
Inactivated whole-virion SARS-CoV-2 vaccines are safe in patients with CLD. Patients with CLD had lower immunologic response to SARS-CoV-2 vaccines than healthy population. The immunogenicity is similarly low in noncirrhotic CLD, compensated cirrhosis, and decompensated cirrhosis.
Keywords: Chronic Liver Diseases, Cirrhosis, Immunogenicity, Safety, SARS-CoV-2 Vaccines
Abbreviations used in this paper: ACE2, angiotensin converting enzyme; AIH, autoimmune hepatitis; ALD, alcoholic liver disease; ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CLD, chronic liver disease; COVID-19, coronavirus disease 2019; IQR, interquartile range; NAFLD, nonalcoholic fatty liver disease; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; ULN, upper limit of normal
What You Need to Know.
Background
Previous studies have assessed safety and immunogenicity of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccines in the population with chronic liver diseases (CLD), but they had relatively small sample sizes, and the results were not compared with that of healthy controls.
Findings
Inactivated whole-virion SARS-CoV-2 vaccines are safe in patients with CLD. Patients with noncirrhotic CLD, compensated cirrhosis, and decompensated cirrhosis all have lower immunologic response to SARS-CoV-2 vaccines than healthy population.
Implications for patient care
The data in this study supported current recommendations for people with CLD to get SARS-CoV-2 vaccination. In the future, patients with CLD might need strengthening strategies, such as a boosting dose of SARS-CoV-2 vaccination, to get better response.
The ongoing coronavirus disease 2019 (COVID-19) pandemic, which is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has led to high morbidity and mortality worldwide.1 Although physical distancing, quarantine, and isolation were effective in limiting the number of people becoming infected during the pandemic in the short term, the absence of herd immunity in the population has left them susceptible to further waves of SARS-CoV-2 infection.2 Chronic liver disease (CLD) is of high prevalence and brings significant medical and economic burden. Global prevalence of nonalcoholic fatty liver disease (NAFLD) is estimated at 24%, whereas globally 75 million people are at risk of alcohol-associated liver disease, and 325 million people are estimated to live with viral hepatitis.3, 4, 5 There were 6.11 million incident cases of CLD that included liver cancer and cirrhosis globally, estimated from the 2017 Global Burden of Disease study.6 Patients with cirrhosis, especially with decompensated cirrhosis, are reported to have an increased risk of SARS-CoV-2 infection and worse outcomes.7, 8, 9
Vaccination is crucial for the restraining of the epidemic and lowering the overall mortality rate, and it has been proven to provide protection from SARS-CoV-2 infection and aggravation of COVID-19.2 Various SARS-CoV-2 vaccines have been proven to induce neutralizing antibody responses and have good safety profiles in healthy adults.10, 11, 12, 13, 14 However, there is not sufficient evidence about safety and immunogenicity of SARS-CoV-2 vaccines in patients with CLD. Previously, researchers have reported decreased immunogenicity of SARS-CoV-2 vaccines in patients under immunocompromised status, including kidney dialysis, using immunosuppression drugs, and organ transplantation including liver transplantation.15, 16, 17 Our previous study has demonstrated good safety and immunogenicity of COVID-19 vaccination in patients with NAFLD.18 Another study showed satisfactory safety but relatively lower immunogenicity in liver transplant recipients and patients with CLD; however, this single-center study had a relatively small sample size, and the results were also not compared with that of healthy controls.19 Currently, the immunogenicity and safety data in patients with CLD are still urgently needed. This prospective, multi-center study includes patients with pre-existing CLD and assesses the safety and immunogenicity of SARS-CoV-2 vaccines in this special population.
Methods
Study Design
In this prospective, multi-center, open-label study, adult participants with pre-existing CLD and healthy volunteers were enrolled from the network of Portal Hypertension Alliance and China-National Medical Center for Infectious Diseases in China. The inclusion criteria of the CLD group included participants over 18 years old, being clinically or pathologically diagnosed with pre-existing CLD, understanding and willing to comply with the study procedures, and providing written informed consent. CLD was defined as diseases of the liver that last over 6 months, including chronic inflammation (hepatitis B, hepatitis C, NAFLD, alcoholic liver disease [ALD], autoimmune hepatitis [AIH], primary biliary cholangitis, and primary sclerosing cholangitis) with or without liver cirrhosis. The healthy control group included participants with normal serological liver function parameters and without history of any liver disease. The exclusion criteria of both groups were pregnancy, lactation, active or known history of SARS-CoV-2 infection, known history of hepatocellular carcinoma or liver transplant, immunosuppressive or immunodeficient state including confirmed HIV infection, and history of receiving systemic immunosuppressants, systemic immunoglobulins, or immunopotentiators within 3 months prior to the day of screening.
All participants received 2 doses of inactivated whole-virion SARS-CoV-2 vaccines (600SU per dose for CoronaVac, 6.5U per dose for BBIBP-CorV and 200WU per dose for WIBP-CorV). The time interval between the first and second SARS-CoV-2 vaccine doses was 3 to 8 weeks, according to the guidance of SARS-CoV-2 vaccinations made by the National Health Commission of the People's Republic of China.20 Basic characteristics (age, sex, body mass index), etiology of liver diseases, presence or absence of cirrhosis and compensation status, comorbidities (diabetes, hypertension, coronary artery disease, arrhythmia, and asthma), and laboratory testing results were obtained from patients’ medical records. Side effects of SARS-CoV-2 vaccines were collected from paper record cards where participants were required to record the injection-site symptoms and systemic symptoms. Serum samples of enrolled subjects were taken at least 14 days after the second dose and tested for SARS-CoV-2 neutralizing antibody. Participants were divided into 3 subgroups based on their cirrhosis and compensation status, where decompensated cirrhosis was defined as cirrhosis with at least 1 episode of ascites, jaundice, hepatic encephalopathy, or variceal bleeding, or with a Child-Pugh of B or C. Abnormality in liver function was defined as any of the following parameter increased over upper limit of normal range including alanine aminotransferase (ALT), aspartate aminotransferase (AST), γ-glutamyl transpeptidase, alkaline phosphatase, total bilirubin, and direct bilirubin.
Written informed consents were obtained before the enrollment. The study protocol and informed consent form were approved by the involved Ethics Committees. This study is registered at ClinicalTrials.gov, NCT04883177.
Antibody Testing
A competitive binding chemiluminescence immunoassays (CLIA) SARS-CoV-2 neutralizing antibody assay (Shenzhen Mindray Bio-Medical Electronics Co, Ltd, China) was used to quantitatively detect neutralizing antibodies to SARS-CoV-2 according to the manufacturer’s instructions.
In the first step, sample, sample treatment solution, and paramagnetic microparticles coated with SARS-CoV-2 antigens are added into a reaction cuvette. After incubation, SARS-CoV-2 neutralizing antibodies present in the sample will bind antigens coated on microparticles. In the second step, angiotensin converting enzyme (ACE2)-alkaline phosphatase (ALP) conjugate is added into the reaction cuvette. After incubation, the ACE2-ALP conjugate competes with the neutralizing antibody in the sample for binding sites of SARS-CoV-2 antigens. Neutralizing antibody or ACE2-ALP conjugate are bound to antigen on the microparticles, which are magnetically captured while other unbound substances are removed by washing. Then, the substrate solution is added to the reaction cuvette. It is catalyzed by ACE2-ALP conjugate in the immunocomplex retained on the microparticles. The result of chemiluminescent reaction is measured as relative light units by a photomultiplier built inside the system. The amount of neutralizing antibody present in the sample is inversely proportional to the relative light units generated during the reaction. The neutralizing antibody concentration is determined via a calibration curve, which is established on an encoded Master Calibration Curve and 3 level product calibrators.
The measuring range are 2.0 to 400.0 AU/mL, and results over 10 AU/mL was considered as evidence of immune response and below 2.0 U/mL as undetectable, according to the instruction book. We defined results over 10.0 AU/mL as positive, and results below 10.0 AU/mL as negative.
Statistical Analysis
Data is displayed as median (interquartile range [IQR]) for the continuous variables and as number of patients and percentage in each group for categorical variables. The Mann-Whitney U test was used for continuous variables, and the Pearson χ2 test or Fisher exact test was used for categorical variables to assess the statistical significance between groups. We fitted binary logistic regression models for univariate and multivariate analysis of factors related to the serological responses. When comparing immunogenic outcomes, we adjusted for factors that were substantially different between patients with CLD and healthy subjects, or among CLD subgroups, using logistic regression or analysis of covariance. Hypothesis testing was 2-sided, and P values of less than 0.05 were considered to be significant. IBM SPSS 24 (IBM Corp, Armonk, NY) and Graphpad Prism version 9.2 were used for statistical analysis.
Patient and Public Involvement Statement
Patients or the public were not involved in the design, conduct, reporting, or dissemination plans of our research.
Results
Participant Characteristics
Between January 2021 and August 2021, a total of 581 participants (437 patients with CLD and 144 healthy volunteers) were enrolled from 15 medical sites in China. The demographic characteristics of participants in CLD groups are shown in Table 1 , and those of the healthy control group in Supplementary Table 1. The median age was 47.0 years (IQR, 38.0-56.0 years) and 35.0 years (IQR, 28.5-41.5 years) in the CLD and healthy control groups, respectively (P < .0001). Among participants with CLD, 384 of 437 patients (87.8%) had chronic viral hepatitis B, whereas others had various etiologies of CLD, including nonalcoholic steatohepatitis, ALD, AIH, primary biliary cholangitis, primary sclerosing cholangitis, etc. Overall, 153 of 437 patients (35.0%) in the CLD group were cirrhotic, 123 of 153 patients (80.4%) with cirrhosis were compensated, and 130 of 141 patients (85.0%) were classified as A in the Child-Pugh classification. In total, 272 of 437 patients (62.2%) had abnormalities in liver function parameters at baseline. As for comorbidities, hypertension (38/437; 8.7%) and diabetes (23/437; 5.3%) were the most common comorbidities in the CLD group (Table 1). Between subgroups, ages were significantly different (median, 43.0 years; IQR, 36.0-53.0 years; median, 51.0 years; IQR, 42.0-58.3 years; and 59.0 years; IQR, 50.8-65.3 years in the noncirrhotic CLD, compensated cirrhosis, and decompensated cirrhosis subgroups, respectively [P < .0001]). The ratio of patients with abnormalities in liver function were significantly higher in the decompensated cirrhotic subgroup (164/284 [57.7%], 84/123 [68.3%], and 24/30 [80.0%] in the noncirrhotic CLD, compensated cirrhosis, and decompensated cirrhosis subgroups, respectively [P = .014]).
Table 1.
Baseline Characteristics of Patients With CLD
| Whole population with CLD (N = 437) | Noncirrhotic CLD (n = 284) | Compensated cirrhosis (n = 123) | Decompensated cirrhosis (n = 30) | P value | |
|---|---|---|---|---|---|
| Age, y | 47.0 (38.0–56.0) | 43.0 (36.0–53.0)a | 51.0 (42.0–58.3)a | 59.0 (50.8–65.3)a | < .0001 |
| Male sex | 278 (63.6) | 176 (62.0) | 84 (68.3) | 18 (60.0) | .435 |
| BMI, kg/m2 | 23.9 (21.7–25.8) | 23.8 (21.5–25.7) | 24.0 (22.3–26.0) | 24.2 (15.9–33.9) | .459 |
| Overweight | 191 (43.7) | 120 (42.3) | 56 (45.5) | 15 (50.0) | .640 |
| Etiology of CLD | |||||
| Hepatitis B | 384 (87.8) | 260 (91.5) | 107 (87.0) | 17 (56.7)a | < .0001 |
| Hepatitis C | 20 (4.6) | 8 (2.8) | 6 (4.9) | 6 (20.0)a | < .0001 |
| NAFLD | 12 (2.7) | 9 (3.2) | 1 (0.8) | 2 (6.7) | .999 |
| ALD | 1 (0.2) | 1 (0.4) | 0 (0.0) | 0 (0.0) | .999 |
| AIH/PBC/PSC | 8 (1.8) | 1 (0.4)a | 5 (4.1) | 2 (6.7)a | < .0001 |
| Other | 12 (2.7) | 5 (1.8) | 4 (3.3) | 3 (10.0) | .999 |
| Cirrhosis | 153 (35.0) | – | – | – | – |
| Compensation, compensated | 123 (80.4) | – | – | – | – |
| Child-Pugh classification | – | – | – | ||
| A | 130/141 (85.0) | – | 111/111 (100.0) | 19/30 (63.3) | < .0001 |
| B | 9/141 (5.9) | – | 0 (0.0) | 9/30 (30.0) | |
| C | 2/141 (1.3) | – | 0 (0.0) | 2/30 (6.7) | |
| Abnormality in liver function | 272 (62.2) | 164 (57.7) | 84 (68.3) | 24 (80.0) | .014 |
| ALT, U/L | 25.5 (18.0–37.2) | 25.0 (18.8–42.0) | 27.0 (18.0–36.0) | 21.8 (15.9–33.9) | .587 |
| AST, U/L | 24.0 (19.5–30.7) | 23.6 (19.1–29.2) | 26.0 (21.0–31.6) | 26.2 (18.8–36.5) | .101 |
| GGT, U/L | 22.0 (15.3–37.0) | 20.7 (14.4–33.0)a | 26.5 (17.8–46.3) | 29.5 (19.0–40.9)a | < .0001 |
| AKP, U/L | 71.7 (57.8–89.0) | 68.0 (55.0–83.6)a | 77.8 (61.9–94.8) | 90.0 (63.5–117.0)a | .001 |
| TBIL, μmol/L | 15.2 (11.5–20.1) | 14.4 (10.9–18.6)a | 17.8 (12.8–21.7) | 21.9 (14.5–31.2)a | < .0001 |
| DBI, μmol/L | 3.3 (2.4–5.3) | 3.0 (2.2–4.9)a | 3.7 (2.7–6.5) | 4.5 (2.9–6.8)a | < .0001 |
| ALB, g/L | 45.5 (43.0–47.9) | 46.0 (43.4–47.9)a | 45.3 (43.0–48.1) | 41.0 (35.9–46.7)a | .002 |
| Comorbidities | 56 (12.8) | 31 (9.9)a | 24 (16.2) | 14 (41.2)a | .001 |
| CAD | 5 (1.1) | 3 (1.1) | 1 (0.8) | 1 (3.3) | .494 |
| HTN | 38 (8.7) | 22 (7.7) | 11 (8.9) | 5 (16.7) | .255 |
| DM | 23 (5.3) | 8 (2.8) | 8 (6.5) | 7 (23.3)a | < .0001 |
| Arrhythmia | 2 (0.5) | 0 (0.0) | 2 (1.6) | 0 (0.0) | .999 |
| Asthma | 1 (0.2) | 1 (0.4) | 0 (0.0) | 0 (0.0) | .999 |
Note: Data are displayed as median (interquartile range) and number (%).
AIH, Autoimmune hepatitis; AKP, alkaline phosphatase; ALB, albumin; ALD, alcoholic liver disease; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; CAD, coronary artery disease; CLD, chronic liver diseases; DBIL, direct bilirubin; DM, diabetes mellitus; GGT, γ-glutamyl transpeptidase; HTN, hypertension; NAFLD, nonalcoholic fatty liver disease; PBC, primary biliary cholangitis; PSC, primary sclerosing cholangitis; TBIL, total bilirubin.
Statistically significantly different from other subgroups.
Vaccine Safety
Seventy participants with CLD (16.0%) reported at least 1 adverse reaction within 14 days of either dose of SARS-CoV-2 vaccination (Table 2 ). There was no significant difference in adverse reactions among subgroups (44 [15.5%], 20 [16.3%], and 6 [20.0%] in the noncirrhotic CLD, compensated cirrhosis, and decompensated cirrhosis subgroups, respectively [P = .812]) (Table 2). Overall, the most common injection site adverse reaction was pain, which was reported in 36 of 437 cases (8.2%). The most commonly reported systematic adverse reaction was fever, which was reported in 9 of 437 cases (2.1%). Additionally, among 164 patients with available laboratory data follow-up visits, there were no cases (0.0%) in the CLD group reporting grade 2 ALT elevation (defined as 2 upper limits of normal [ULN]<ALT ≤ 5 ULN), 3 of 164 participants (1.8%) reported grade 3 ALT elevation (defined as ALT >5 ULN), and 4 of 164 participants (2.4%) reported grade 2 AST elevation (defined as 2 ULN < AST ≤ 5 ULN) after the whole schedule of SARS-CoV-2 vaccination (Supplementary Table 2). One of the 3 participants with grade 3 abnormalities was hospitalized for developing a trend of acute liver failure in the follow-up visits, and this was considered potentially related to SARS-CoV-2 vaccination, whereas the rest received related treatment and took follow-up visits as outpatients. Detailed information on the 3 participants with grade 3 ALT elevation are listed in Supplementary Table 3.
Table 2.
Adverse Reactions After Either Dose of SARS-CoV-2 Vaccines
| Whole population with CLD (N = 437) | Noncirrhotic CLD (n = 284) | Compensated cirrhosis (n = 123) | Decompensated cirrhosis (n = 30) | |
|---|---|---|---|---|
| Solicited adverse reactions within 0 to 14 days | ||||
| Any | 70 (16.0) | 44 (15.5) | 20 (16.3) | 6 (20.0) |
| Grade 1 | 64 (14.6) | 39 (13.7) | 20 (16.3) | 5 (16.7) |
| Grade 2 | 6 (1.4) | 5 (1.8) | 0 (0.0) | 1 (3.3) |
| Injection site adverse reactions | ||||
| Pain | 36 (8.2) | 25 (8.8) | 10 (8.1) | 1 (3.3) |
| Grade 1 | 34 (7.8) | 23 (8.1) | 10 (8.1) | 1 (3.3) |
| Grade 2 | 2 (0.5) | 2 (0.7) | 0 (0.0) | 0 (0.0) |
| Swelling | 4 (0.9) | 3 (1.1) | 1 (0.8) | 0 (0.0) |
| Grade 1 | 4 (0.9) | 3 (1.1) | 1 (0.8) | 0 (0.0) |
| Induration | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Erythema | 2 (0.5) | 1 (0.4) | 1 (0.8) | 0 (0.0) |
| Grade 1 | 2 (0.5) | 1 (0.4) | 1 (0.8) | 0 (0.0) |
| Pruritus | 2 (0.5) | 1 (0.4) | 0 (0.0) | 1 (3.3) |
| Grade 1 | 2 (0.5) | 1 (0.4) | 0 (0.0) | 1 (3.3) |
| Systematic adverse reactions | ||||
| Fever | 9 (2.1) | 6 (2.1) | 1 (0.8) | 2 (6.7) |
| Grade 1 | 7 (1.6) | 5 (1.8) | 1 (0.8) | 1 (3.3) |
| Grade 2 | 2 (0.5) | 1 (0.4) | 0 (0.0) | 1 (3.3) |
| Cough | 2 (0.5) | 1 (0.4) | 1 (0.8) | 0 (0.0) |
| Grade 1 | 2 (0.5) | 1 (0.4) | 1 (0.8) | 0 (0.0) |
| Vertigo | 4 (0.9) | 1 (0.4) | 3 (2.4) | 0 (0.0) |
| Grade 1 | 3 (0.7) | 0 (0.0) | 3 (2.4) | 0 (0.0) |
| Grade 2 | 1 (0.2) | 1 (0.4) | 0 (0.0) | 0 (0.0) |
| Nausea | 3 (0.7) | 1 (0.4) | 1 (0.8) | 1 (3.3) |
| Grade 1 | 3 (0.7) | 1 (0.4) | 1 (0.8) | 1 (3.3) |
| Vomiting | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Grade 1 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Myalgia | 3 (0.7) | 2 (0.7) | 0 (0.0) | 1 (3.3) |
| Grade 1 | 3 (0.7) | 2 (0.7) | 0 (0.0) | 1 (3.3) |
| Arthralgia | 1 (0.2) | 0 (0.0) | 1 (0.8) | 0 (0.0) |
| Grade 1 | 1 (0.2) | 0 (0.0) | 1 (0.8) | 0 (0.0) |
| Fatigue | 8 (1.8) | 6 (2.1) | 2 (1.6) | 0 (0.0) |
| Grade 1 | 7 (1.6) | 5 (1.8) | 2 (1.6) | 0 (0.0) |
| Grade 2 | 1 (0.2) | 1 (0.4) | 0 (0.0) | 0 (0.0) |
| Hypersensitivity | 1 (0.2) | 1 (0.4) | 0 (0.0) | 0 (0.0) |
| Grade 1 | 1 (0.2) | 1 (0.4) | 0 (0.0) | 0 (0.0) |
| Anorexia | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Dyspnea | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Syncope | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Pharyngalgia | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
Note: Data are displayed as number (%), representing the total number of participants who had adverse reactions (ie, adverse events related to vaccination).
CLD, Chronic liver disease; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
SARS-CoV-2 Vaccination Immunogenicity
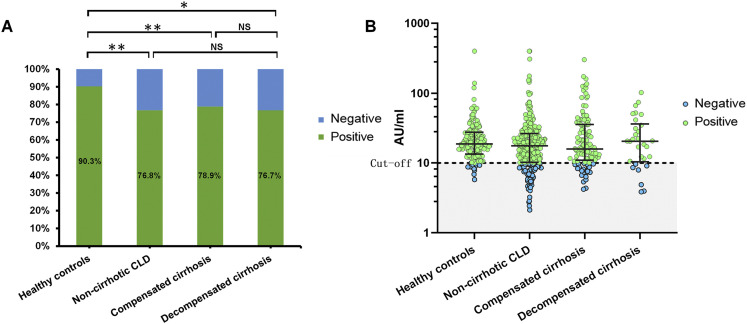
The positive rate of SARS-CoV-2 neutralizing antibodies were significantly lower in participants with CLD (338/437 [77.3%] in the CLD group and 130/144 [90.3%] in the healthy control group; P = .001). After adjusting for age, gender, and body mass index, the difference was still statistically significant (P = .035). The positive rate was similar in participants with noncirrhotic CLD, compensated cirrhosis, and decompensated cirrhosis (218/284 [76.8%], 97/123 [78.9%], and 23/30 [76.7%], respectively; P = .894) (Figure 1A). After adjusting for age, chronic hepatitis C, and diabetes, the difference was still not statistically significant (P = .545). The neutralizing antibody concentration were 17.7 AU/mL (IQR, 10.3-26.5 AU/mL) in the noncirrhotic CLD group, 15.9 AU/mL (IQR, 11.0-35.6 AU/mL) in the compensated cirrhotic group, 20.5 AU/mL (IQR, 10.4-36.4 AU/mL) in the decompensated cirrhotic group, and 18.8 AU/mL (IQR, 13.4-27.7 AU/mL) in the healthy control group (P = .137) (Figure 1B).
Figure 1.
Serological antibody response of SARS-CoV-2 vaccines in patients with chronic liver diseases. Positive rates and concentrations of neutralizing antibodies to SARS-CoV-2 induced after the whole schedule of SARS-CoV-2 vaccination in participants with noncirrhotic CLD, compensated cirrhosis, decompensated cirrhosis, and healthy controls. Concentrations over 10.0 AU/mL were considered as positive, and concentrations below 10.0 AU/mL as negative. NS, Non-significant. ∗P value < .05; ∗∗P value < .01.
Factors Related to Serological Response Among Patients With Chronic Liver Diseases
Univariate and multivariate analysis of factors associated with negative serological response of SARS-CoV-2 vaccines was conducted in the CLD group (Table 3 ). Male gender was suggested to be an independent risk factor for negative serological response to SARS-CoV-2 vaccination (odds ratio, 1.89; 95% confidence interval, 1.12-3.90; P = .017) after taking age, cirrhosis, whether overweight, and compensation status into consideration.
Table 3.
Factors for Negative Serological Response to SARS-CoV-2 Vaccination in Patients With CLD
| Positive (n = 377) | Negative (n = 119) | Univariate analysis |
Multivariate analysis |
|||
|---|---|---|---|---|---|---|
| OR | P value | OR | P value | |||
| Age, y | 46.0 (37.0–56.0) | 49.0 (39.0–57.0) | – | .299 | – | .161 |
| Male sex | 205 (60.7) | 73 (73.7) | 1.82 (1.11–3.00) | .018 | 1.86 (1.12–3.90) | .017 |
| BMI, kg/m2 | 23.8 (21.5–25.8) | 24.3 (22.2–25.9) | – | .293 | ||
| Overweight | 139 (41.1) | 52 (52.5) | 1.58 (1.01–2.48) | .045 | 1.47 (0.93–2.32) | .101 |
| Etiology of CLD (viral hepatitis/ NAFLD/ALD/AIH/PBC/PSC) | – | – | – | .393 | ||
| Cirrhosis and compensation statusa | ||||||
| Noncirrhotic CLD | 218 (64.5) | 66 (66.7) | – | – | – | – |
| Compensated cirrhosis | 97 (28.7) | 26 (26.3) | 1.00 (0.41–2.45) | .991 | 0.81 (0.32–2.07) | .664 |
| Decompensated cirrhosis | 23 (6.8) | 7 (7.1) | 1.13 (0.44–2.94) | .793 | 1.07 (0.41–2.82) | .894 |
| Chronic hepatitis B | ||||||
| HBeAg status, positive | 67 (22.4) | 26 (31.0) | 1.60 (0.91–2.81) | .103 | ||
| HBV DNA status, detectable (%) | 66 (22.1) | 20 (23.8) | 1.08 (0.60–1.95) | .801 | ||
| Under antiviral therapy | 169 (56.5) | 43 (51.2) | 0.76 (0.32–1.82) | .542 | ||
| Different antiviral regimen | – | – | – | .694 | ||
| Abnormality in liver function at baseline | 207 (61.2) | 65 (65.6) | 1.30 (0.79–2.14) | .300 | ||
| ALT, U/L | 25.6 (17.9–38.2) | 24.0 (18.5–37.1) | – | .649 | ||
| AST, U/L | 24.0 (19.5–31.1) | 24.0 (19.8–29.8) | – | .944 | ||
| GGT, U/L | 22.0 (15.0–38.0) | 21.0 (16.1–34.2) | – | .845 | ||
| AKP, U/L | 69.9 (57.0–89.0) | 75.0 (58.3–89.5) | – | .688 | ||
| TBIL, μmol/L | 14.6 (11.3–19.9) | 16.9 (12.9–21.9) | – | .083 | ||
| DBIL, μmol/L | 3.2 (2.3–5.1) | 3.8 (2.7–5.9) | – | .221 | ||
| ALB, g/L | 45.6 (43.0–47.6) | 45.3 (42.8–48.8) | – | .712 | ||
Note: Data are displayed as median (interquartile range) and number (%).
AIH, Autoimmune hepatitis; AKP, alkaline phosphatase; ALB, albumin; ALD, alcoholic liver disease; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; CLD, chronic liver diseases; DBIL, direct bilirubin; GGT, γ-glutamyl transpeptidase; HBV, hepatitis B virus; NAFLD, nonalcoholic fatty liver disease; OR, odds ratio; PBC, primary biliary cholangitis; PSC, primary sclerosing cholangitis; TBIL, total bilirubin.
OR of compensated and decompensated cirrhosis were compared with noncirrhotic CLD.
Discussion
Previous studies had provided us with sufficient data about safety and immunogenicity of SARS-CoV-2 vaccines in healthy adults, showing a satisfactory result.10, 11, 12 However, the immune response data in the population with CLD is still insufficient; previous studies either had a relatively small sample size or the results were not compared with that of healthy controls. Our multi-center study evaluated the safety and immunogenicity of SARS-CoV-2 vaccines in a real-world population with CLD, providing further evidence for SARS-CoV-2 vaccination in patients with CLD and those with different statuses of cirrhosis and compensation.
Our study indicated that the whole schedule (2 doses) of inactivated whole-virion SARS-CoV-2 vaccination is relatively safe in patients with CLD. All adverse symptoms were not severe and mostly transient, and injection site pain was the most reported symptom. As for laboratory indicator abnormalities, 3 patients had significant aminopherase elevation, all of whom had elevated aminopherase at baseline. Only 1 of them was judged as having a severe adverse event, in a patient who developed a trend of acute liver failure and had hospitalization in the follow-up. However, it was uncertain whether this severe adverse event was related to the SARS-CoV-2 vaccination, because the patient had a history of discontinuating anti-hepatitis B virus agents before SARS-CoV-2 vaccination. Overall, the safety was similarly good in patients with noncirrhotic CLD, compensated cirrhosis, and decompensated cirrhosis. The result also suggested that patients with baseline liver function abnormalities may need to monitor serum indicators of liver function after SARS-CoV-2 vaccination, but the strategy of monitoring and follow-up plan need further research. As for the healthy control group, the SARS-CoV-2 inactivated vaccines used in this study had previously reported satisfactory safety results in healthy adults.10 , 11 In this research, participants in the healthy control group were asked to make unsolicited reports to investigators if there was any adverse event, and no severe adverse events were reported, consistent with previous studies.
Patients with CLD had lower immunologic response to SARS-CoV-2 vaccines than healthy subjects. In the subgroup analysis, we observed similar immunologic response in the patients with noncirrhotic CLD, compensated cirrhosis, and decompensated cirrhosis. These results are consistent with clinical phenomenon and previous studies, where patients with liver impairment had worse response to other vaccines such as hepatitis B virus vaccines.21 , 22 Decreased responses to SARS-CoV-2 vaccination might be related to the impairment in immunity in the population with CLD.23 CLD and fibrosis impair the synthesis of innate immunity proteins and pattern recognition receptors (although lower serum levels of these proteins are only evident in patients with advanced cirrhosis because of the large functional reserve of the liver), and also affect B lymphocytes and T lymphocytes in both absolute counts and functions through a variety of mechanisms such as down-regulation of co-stimulation markers, loss of memory cells, and T cell exhaustion.23, 24, 25, 26, 27 In the future, patients with CLD might need a boosting dose of SARS-CoV-2 vaccination to get a better response.
Our study suggested that male gender was an independent risk factor for negative serological response to SARS-CoV-2 vaccination, which is consistent with previous research about other vaccines, where male gender had been implicated in the poor vaccination responsiveness in the general population.28, 29, 30, 31 A number of hypotheses on the relationship between sex differences in the human immune system and mechanisms involved in the antibody response to vaccination have been made previously by scientists, such as testosterone-modulating genes involving lipid metabolism and antibody-neutralizing response, but the underlying mechanisms are still largely not understood.32 This may further provide evidence for screening the population with higher risk of negative vaccination responsiveness and making recommendations of strengthening strategies.
The limitations of our study include a relatively small sample size of the decompensated cirrhosis subgroup and a short follow-up period. Few participants had severe impaired liver function at baseline in our study, even in the decompensated cirrhosis subgroup. Research with larger sample size and more various liver function status are required in the future. Patients receiving systemic immunosuppressants were not included in this study, which excluded a relevant number of patients with CLD, such as those patients with AIH under therapy, and this special population group also needs further investigation in the future. In addition, the population of patients with CLD in this study were typical in the East (mainly patients with chronic hepatitis B), and more patients with NAFLD or ALD need to be further observed. Moreover, we did not test for T-cell mediated response or other additional immunologic tests, which would be designed and conducted in next steps. Lastly, the findings of this study may not be extrapolated to mRNA vaccines, which can induce a stronger antibody response, and further research investigating efficacy of a variety of vaccines, regarding COVID-19 morbidity and mortality following the vaccination in patients with CLD are required in the future.
Conclusions
In conclusion, inactivated whole-virion SARS-CoV-2 vaccines are safe in patients with CLD, including patients with compensated and decompensated cirrhosis, supporting current recommendations for patients with CLD. Our findings raise a concern regarding lower immunologic response to SARS-CoV-2 vaccines in patients with CLD, and might help make recommendations of strengthening strategies in the future.
Acknowledgment
The authors thank Shenzhen Mindray Bio-Medical Electronics Co., Ltd for cordially providing SARS-CoV-2 neutralizing antibody chemiluminescent immunoassay (CLIA) reagents.
CRediT Authorship Contribution
Jingwen Ai (Formal analysis: Lead; Investigation: Equal; Writing – original draft: Lead)
Jitao Wang (Data curation: Equal; Investigation: Equal; Validation: Equal; Writing – original draft: Equal; Writing – review & editing: Equal)
Dengxiang Liu (Data curation: Equal; Formal analysis: Equal; Investigation: Equal)
Huiling Xiang (Data curation: Equal; Investigation: Equal; Project administration: Equal)
Ying Guo (Data curation: Equal; Investigation: Equal; Project administration: Equal)
Jiaojian Lv (Data curation: Equal; Investigation: Equal; Project administration: Equal)
Qiran Zhang (Data curation: Equal; Formal analysis: Equal; Project administration: Equal; Writing – original draft: Equal)
Jinlong Li (Data curation: Equal; Investigation: Equal; Project administration: Equal)
Xiaochong Zhang (Data curation: Equal; Investigation: Equal; Project administration: Equal)
Qianqian Li (Investigation: Equal; Project administration: Equal)
Jing Liang (Investigation: Equal; Project administration: Equal)
Xiaoqing Guo (Project administration: Equal)
Yinong Feng (Investigation: Equal; Project administration: Supporting)
Luxiang Liu (Investigation: Equal; Project administration: Supporting)
Xuying Zhang (Investigation: Supporting; Project administration: Equal)
Wei Qin (Investigation: Equal; Project administration: Supporting)
Xiaodong Wang (Investigation: Supporting; Project administration: Equal)
Wei Rao (Investigation: Supporting; Project administration: Equal)
Qun Zhang (Investigation: Equal; Project administration: Equal)
Qiuju Tian (Data curation: Equal; Investigation: Equal; Project administration: Supporting)
Yanliang Zhang (Data curation: Equal; Investigation: Supporting)
Faren Xie (Data curation: Supporting; Project administration: Equal)
Shujun Jiang (Project administration: Supporting; Validation: Equal)
Yan Yan (Investigation: Equal; Project administration: Supporting)
Yuanwang Qiu (Data curation: Supporting; Project administration: Equal)
Hangyuan Wu (Data curation: Equal; Project administration: Supporting)
Zhiyun Hou (Investigation: Supporting; Project administration: Supporting)
Nina Zhang (Data curation: Supporting; Investigation: Supporting)
Aiguo Zhang (Investigation: Equal; Project administration: Supporting)
Jiansong Ji (Investigation: Supporting; Project administration: Equal)
Jie Yang (Investigation: Supporting; Project administration: Supporting)
Jiansheng Huang (Funding acquisition: Supporting; Validation: Supporting)
Zhongwei Zhao (Formal analysis: Supporting; Project administration: Supporting)
Ye Gu (Investigation: Supporting; Resources: Supporting)
Li Bian (Methodology: Equal; Project administration: Supporting)
Zhen Zhang (Investigation: Equal; Project administration: Supporting)
Shengqiang Zou (Methodology: Supporting; Project administration: Supporting)
Hailei Ji (Investigation: Supporting; Project administration: Supporting)
Guohong Ge (Formal analysis: Supporting; Investigation: Supporting)
Xiufang Du (Investigation: Supporting; Project administration: Supporting)
Aifang Hou (Data curation: Supporting; Project administration: Supporting)
Ying Zhu (Formal analysis: Supporting; Project administration: Supporting)
Qingwei Cong (Investigation: Supporting; Project administration: Supporting)
Juan Xu (Investigation: Supporting; Project administration: Supporting)
Hongmei Zu (Investigation: Supporting; Project administration: Supporting)
Yun Wang (Formal analysis: Supporting; Project administration: Supporting)
Zhaolan Yan (Investigation: Supporting; Resources: Supporting)
Xiaosong Yan (Project administration: Supporting; Resources: Supporting)
Yangzhen Bianba (Investigation: Supporting; Project administration: Supporting)
Qu Ci (Investigation: Supporting; Project administration: Supporting)
Liting Zhang (Investigation: Supporting; Project administration: Supporting)
Shiying Yang (Investigation: Supporting; Project administration: Supporting)
Xiaoqin Gao (Investigation: Supporting; Project administration: Supporting; Validation: Supporting)
Li Zhong (Formal analysis: Supporting; Project administration: Supporting)
Song He (Formal analysis: Supporting; Project administration: Supporting)
Chuan Liu (Formal analysis: Supporting; Project administration: Supporting)
Yifei Huang (Formal analysis: Supporting; Resources: Supporting)
Yanna Liu (Formal analysis: Supporting; Project administration: Supporting)
Dan Xu (Investigation: Supporting; Project administration: Supporting)
Qingliang Zhu (Investigation: Supporting; Project administration: Supporting)
Xinxin Xu (Investigation: Supporting; Project administration: Supporting)
Muhan Lv (Investigation: Supporting; Project administration: Supporting)
Wenhong Zhang (Conceptualization: Lead; Supervision: Equal; Writing – review & editing: Equal)
Xiaolong Qi (Conceptualization: Equal; Supervision: Lead; Writing – review & editing: Lead)
Footnotes
Conflicts of interest The authors disclose no conflicts.
Note: To access the supplementary material accompanying this article, visit the online version of Clinical Gastroenterology and Hepatology at www.cghjournal.org, and at http://doi.org/10.1016/j.cgh.2021.12.022.
Supplementary Material
Supplementary Table 1.
Baseline Characteristics of Healthy Controls
| Healthy controls (n = 144) | |
|---|---|
| Age, y | 35.0 (28.5–41.5) |
| Male sex | 45 (31.3) |
| BMI, kg/m2 | 22.6 (20.0-25.5) |
| Overweight | 55 (38.7) |
Note: Data are displayed as median (interquartile range) and number (%).
BMI, Body mass index.
Supplementary Table 2.
Laboratory Abnormalities in Patients With CLD After SARS-CoV-2 Vaccination
| Whole population with CLD (n = 164) | Noncirrhotic CLD (n = 82) | Compensated cirrhosis (n = 59) | Decompensated cirrhosis (n = 23) | |
|---|---|---|---|---|
| ALT | ||||
| Grade 1 | 32 (19.5) | 17 (20.7) | 11 (18.6) | 4 (17.4) |
| Grade 2 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Grade 3 | 3 (1.8) | 2 (2.4) | 1 (1.7) | 0 (0.0) |
| AST | ||||
| Grade 1 | 18 (11.0) | 5 (6.1) | 7 (11.9) | 6 (26.1) |
| Grade 2 | 4 (2.4) | 2 (2.4) | 1 (1.7) | 1 (4.3) |
| Grade 3 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
Note: Data are displayed as number (%).
Note: Grade 1: 1 ULN < ALT or AST ≤ 2 ULN; Grade 2: 2 ULN < ALT or AST ≤ 5 ULN; Grade 3: ALT or AST > 5 ULN.
ALT, Alanine aminotransferase; AST, aspartate aminotransferase; CLD, chronic liver disease; ULN, upper limits of normal.
Supplementary Table 3.
Information on Patients With Grade 3 ALT Elevation
| Basic information | |||
|---|---|---|---|
| Patient ID | Sex | Age, y | BMI, kg/m2 |
| 149 | Male | 31 | 29.3 |
| 387 | Male | 31 | 20.3 |
| 473 | Male | 31 | 24.8 |
| Detailed information about CLD | |||||
|---|---|---|---|---|---|
| Patient ID | Cirrhosis and compensation | Etiology of CLD | HBeAg status | HBV-DNA status | Anti-viral drugs |
| 149 | Noncirrhotic CLD | Chronic hepatitis B | Positive | Detectable | Nonea |
| 387 | Compensated cirrhosis | Chronic hepatitis B | Positive | Detectable | TDF |
| 473 | Noncirrhotic CLD | Chronic hepatitis B | Positive | Detectable | ETV |
| Baseline liver function | |||||||
|---|---|---|---|---|---|---|---|
| Patient ID | ALT, U/L | AST, U/L | GGT, U/L | AKP, U/L | TBIL, μmol/L | DBIL, μmol/L | ALB, g/L |
| 149 | 167 | 298 | 131 | 102 | 54.4 | 31.6 | 38.7 |
| 387 | 80 | 75 | 55 | 40 | 20.5 | 2.3 | 40 |
| 473 | 360 | 112 | 56 | 81 | 8.4 | 2.2 | 44.7 |
| Liver function after 2 doses of SARS-CoV-2 vaccination | |||||||
|---|---|---|---|---|---|---|---|
| Patient ID | ALT, U/L | AST, U/L | GGT, U/L | AKP, U/L | TBIL, μmol/L | DBIL, μmol/L | ALB, g/L |
| 149 | 498 | 192 | 78 | 108 | 38 | 10.1 | 44.4 |
| 387 | 377.5 | 122.7 | 65 | 45 | 23.8 | 3.6 | 40 |
| 473 | 219 | 120 | 92 | 89 | 19.4 | 6.2 | 42.1 |
AKP, Alkaline phosphatase; ALB, albumin; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; CLD, chronic liver disease; DBIL, direct bilirubin; ETV, entecavir; HBV, hepatitis B virus; GGT, γ-glutamyl transpeptidase; TBIL, total bilirubin; TDF, tenofovir disoproxil fumarate.
Patient 149 discontinued TDF against doctor’s administration.
References
- 1.World Health Organiztion coronavirus (COVID-19) dashboard. https://covid19.who.int/ Available at: . Accessed October 1, 2021.
- 2.Carvalho T., Krammer F., Iwasaki A. The first 12 months of COVID-19: a timeline of immunological insights. Nat Rev Immunol. 2021;21:245–256. doi: 10.1038/s41577-021-00522-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Younossi Z., Anstee Q.M., Marietti M., et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15:11–20. doi: 10.1038/nrgastro.2017.109. [DOI] [PubMed] [Google Scholar]
- 4.Asrani S.K., Devarbhavi H., Eaton J., et al. Burden of liver diseases in the world. J Hepatol. 2019;70:151–171. doi: 10.1016/j.jhep.2018.09.014. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization hepatitis topics. https://www.who.int/health-topics/hepatitis#tab=tab_1 Available at:
- 6.Paik J.M., Golabi P., Younossi Y., et al. Changes in the global burden of chronic liver diseases from 2012 to 2017: the growing impact of NAFLD. Hepatology. 2020;72:1605–1616. doi: 10.1002/hep.31173. [DOI] [PubMed] [Google Scholar]
- 7.Marjot T., Moon A.M., Cook J.A., et al. Outcomes following SARS-CoV-2 infection in patients with chronic liver disease: an international registry study. J Hepatol. 2021;74:567–577. doi: 10.1016/j.jhep.2020.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sarin S.K., Choudhury A., Lau G.K., et al. APASL COVID Task Force APASL COVID Liver Injury Spectrum Study (APCOLIS Study- NCT 04345640). Pre-existing liver disease is associated with poor outcome in patients with SARS CoV2 infection: the APCOLIS Study (APASL COVID-19 Liver Injury Spectrum Study) Hepatol Int. 2020;14:690–700. doi: 10.1007/s12072-020-10072-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iavarone M., D’Ambrosio R., Soria A., et al. High rates of 30-day mortality in patients with cirrhosis and COVID-19. J Hepatol. 2020;73:1063–1071. doi: 10.1016/j.jhep.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xia S., Zhang Y., Wang Y., et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: a randomised, double-blind, placebo-controlled, phase 1/2 trial. Lancet Infect Dis. 2021;21:39–51. doi: 10.1016/S1473-3099(20)30831-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang Y., Zeng G., Pan H., et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18-59 years: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis. 2021;21:181–192. doi: 10.1016/S1473-3099(20)30843-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ramasamy M.N., Minassian A.M., Ewer K.J., et al. Oxford COVID Vaccine Trial Group Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): a single-blind, randomised, controlled, phase 2/3 trial. Lancet. 2021;396:1979–1993. doi: 10.1016/S0140-6736(20)32466-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walsh E.E., Frenck R.W., Jr., Falsey A.R., et al. Safety and immunogenicity of two RNA-based Covid-19 vaccine candidates. N Engl J Med. 2020;383:2439–2450. doi: 10.1056/NEJMoa2027906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu F.C., Li Y.H., Guan X.H., et al. Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine: a dose-escalation, open-label, non-randomised, first-in-human trial. Lancet. 2020;395:1845–1854. doi: 10.1016/S0140-6736(20)31208-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boyarsky B.J., Werbel W.A., Avery R.K., et al. Immunogenicity of a single dose of SARS-CoV-2 messenger RNA vaccine in solid organ transplant recipients. JAMA. 2021;325:1784–1786. doi: 10.1001/jama.2021.4385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zitt E., Davidovic T., Schimpf J., et al. The safety and immunogenicity of the mRNA-BNT162b2 SARS-CoV-2 vaccine in hemodialysis patients. Front Immunol. 2021;12:704773. doi: 10.3389/fimmu.2021.704773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rabinowich L., Grupper A., Baruch R., et al. Low immunogenicity to SARS-CoV-2 vaccination among liver transplant recipients. J Hepatol. 2021;75:435–438. doi: 10.1016/j.jhep.2021.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang J., Hou Z., Liu J., et al. Safety and immunogenicity of COVID-19 vaccination in patients with non-alcoholic fatty liver disease (CHESS2101): a multicenter study. J Hepatol. 2021;75:439–441. doi: 10.1016/j.jhep.2021.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thuluvath P.J., Robarts P., Chauhan M. Analysis of antibody responses after COVID-19 vaccination in liver transplant recipients and those with chronic liver diseases. J Hepatol. 2021;75:1434–1439. doi: 10.1016/j.jhep.2021.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.National Health Commission of the People's Republic of China Guidance of SARS-CoV-2 vaccination (First version) Chinese J Clin Infect Dis. 2021;14:89–90. [Google Scholar]
- 21.Aggeletopoulou I., Davoulou P., Konstantakis C., et al. Response to hepatitis B vaccination in patients with liver cirrhosis. Rev Med Virol. 2017;27 doi: 10.1002/rmv.1942. [DOI] [PubMed] [Google Scholar]
- 22.Keeffe E.B., Iwarson S., McMahon B.J., et al. Safety and immunogenicity of hepatitis A vaccine in patients with chronic liver disease. Hepatology. 1998;27:881–886. doi: 10.1002/hep.510270336. [DOI] [PubMed] [Google Scholar]
- 23.Albillos A., Lario M., Álvarez-Mon M. Cirrhosis-associated immune dysfunction: distinctive features and clinical relevance. J Hepatol. 2014;61:1385–1396. doi: 10.1016/j.jhep.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 24.Dhanda A.D., Collins P.L. Immune dysfunction in acute alcoholic hepatitis. World J Gastroenterol. 2015;21:11904–11913. doi: 10.3748/wjg.v21.i42.11904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou L., He R., Fang P., et al. Hepatitis B virus rigs the cellular metabolome to avoid innate immune recognition. Nat Commun. 2021;12:98. doi: 10.1038/s41467-020-20316-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gao B., Jeong W.I., Tian Z. Liver: An organ with predominant innate immunity. Hepatology. 2008;47:729–736. doi: 10.1002/hep.22034. [DOI] [PubMed] [Google Scholar]
- 27.Schirren C.A., Jung M.C., Zachoval R., et al. Analysis of T cell activation pathways in patients with liver cirrhosis, impaired delayed hypersensitivity and other T cell-dependent functions. Clin Exp Immunol. 1997;108:144–150. doi: 10.1046/j.1365-2249.1997.d01-985.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wiedermann U., Garner-Spitzer E., Wagner A. Primary vaccine failure to routine vaccines: why and what to do? Hum Vaccin Immunother. 2016;12:239–243. doi: 10.1080/21645515.2015.1093263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang S., Tian G., Cui Y., et al. Factors influencing immunologic response to hepatitis B vaccine in adults. Sci Rep. 2016;6:27251. doi: 10.1038/srep27251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fischinger S., Boudreau C.M., Butler A.L., et al. Sex differences in vaccine-induced humoral immunity. Semin Immunopathol. 2019;41:239–249. doi: 10.1007/s00281-018-0726-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fehervari Z. Vaccine sex differences. Nat Immunol. 2019;20:111. doi: 10.1038/s41590-018-0310-0. [DOI] [PubMed] [Google Scholar]
- 32.Furman D., Hejblum B.P., Simon N., et al. Systems analysis of sex differences reveals an immunosuppressive role for testosterone in the response to influenza vaccination. Proc Natl Acad Sci U S A. 2014;111:869–874. doi: 10.1073/pnas.1321060111. [DOI] [PMC free article] [PubMed] [Google Scholar]