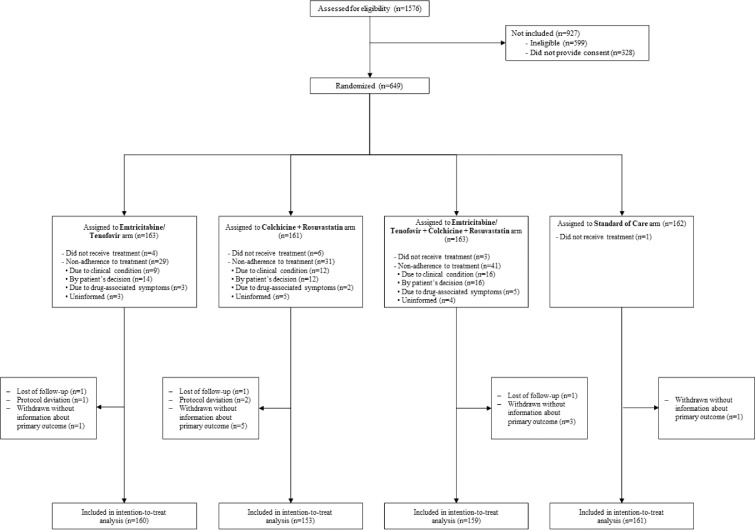
Figure 1.
Enrolment, Randomisation, and Inclusion in the intention-to-treat analysis.
1576 patients were seen in the six hospitals; 599 (38%) had some exclusion criteria, mainly chronic use of statins in 582. Overall, 994 patients were invited to participate in the study, 328 (33%) refused to sign the informed consent, 649 were randomised and assigned to one of four arms of treatment. Of them, 3 subjects did not meet protocol selection criteria, 3 subjects were lost of follow-up and 10 patients withdrew; consequently, the primary outcome was unknown in 13 patients, so they were not included in the analysis. Finally, 633 patients were considered in the modified ITT analysis.