Abstract
Potent synthetic opioids are an important cause of death in the United States' opioid epidemic, and a breathing stimulant may have utility in treating opioid overdose. We hypothesized that sufentanil-induced respiratory depression may be reversed by breathing stimulant administration. Using nose-only plethysmography and arterial blood analysis, we compared effects of several breathing stimulants in reversing sufentanil-induced respiratory depression in conscious rats. We studied taltirelin (1 mg/kg i.v.), PKTHPP (5 mg/kg i.v.), CX717 (30 mg/kg i.v.), BIMU8 (1 mg/kg i.v.), A85380 (30 μg/kg i.v.), and 8-hydroxy-2-(di-n-propylamino)tetralin (8-OH-DPAT) (150 μg/kg i.v./i.m.) and used sufentanil (10 μg/kg i.v.). By plethysmography (in % baseline, mean ± S.E.M.), taltirelin restored ventilation in sufentanil-treated rats (from 50 ± 5% to 102 ± 8%) by increased breathing rates (from 80 ± 4% to 160 ± 12%). By arterial blood analysis, however, taltirelin did not correct hypoxia, decreased hypercarbia only after 45 minutes, and worsened metabolic acidosis (base excess from +0 ± 1 to −7 ± 1 mEq/l). Additionally, taltirelin increased exhaled carbon dioxide, an estimate of oxygen consumption, by up to 64%. PKTHPP, CX717, BIMU8, and A85380 failed to significantly change ventilation or arterial blood values in sufentanil-treated rats. 8-OH-DPAT, however, improved ventilation (from 54 ± 8% to 92 ± 10%), reversed hypercarbia (from 64 ± 6 to 47 ± 2 mmHg), and shortened time to righting from 43 ± 4 to 15 ± 1 minutes in sufentanil-treated rats placed supine. Taltirelin has limited therapeutic potential, as its ventilatory effects are offset by metabolic acidosis, possibly from increased oxygen consumption. At the doses studied, PKTHPP, CX717, BIMU8, and A85380 have limited effects in reversing sufentanil-induced respiratory depression; 8-OH-DPAT, however, warrants further study.
Significance Statement
Respiratory depression is an important cause of death after potent synthetic opioid overdose. 8-Hydroxy-2-(di-n-propylamino)tetralin or related compounds may be useful in treating respiratory depression as caused by potent synthetic opioids.
Introduction
Opioids are highly effective drugs used in pain management; however, they impose a range of undesired and sometimes lethal side effects, including sedation, respiratory depression, skeletal muscle rigidity, and impaired coordination (Dahan et al., 2010). Because they cause euphoria, opioids are also addictive and a common drug of abuse (Volkow et al., 2016).
There are three general opioid classes: 1) natural opiates found in opium (e.g., morphine and codeine); 2) semisynthetic, chemical derivatives of opiates (e.g., heroin, hydromorphone); and 3) fully synthetic (e.g., fentanyl and its many derivatives). Fentanyl and several derivatives (e.g., sufentanil, alfentanil, and remifentanil) are widely used in anesthesia and pain management and are appreciated for their rapid onset and transcutaneous and transmucosal bioavailability. However, because of their marked clinical potency and ease of chemical synthesis and derivatization, fentanyl and its many derivatives have become endemic in the illicit drug supply fueling the ongoing United States opioid epidemic health crisis and are a major contributor to overdose deaths (Wilson et al., 2020). Potent synthetic opioids are also recognized as a chemical threat that, through weaponization (e.g., aerosolization) or accidental release, might cause mass casualty (Tsou et al., 1989; Schiermeier, 2002; Doucette, 2017; Shafer, 2019; Yeung et al., 2020).
Naloxone, an opioid antagonist drug, provides rapid and reliable reversal of opioid effects, such as respiratory depression, when administered via multiple routes (e.g., intravenous, nasal, and intramuscular). Because of its mechanism of action and short duration of action, however, large and repeat naloxone dosing followed by continuous administration is sometimes required to revive an individual suffering potent synthetic opioid overdose, which can complicate medical management and stress healthcare resources (Sutter et al., 2017; Uddayasankar et al., 2018). Because respiratory depression is the primary cause of opioid overdose death, when naloxone therapy is insufficient, breathing stimulant drugs may potentially reverse opioid-induced respiratory depression by themselves or in tandem with naloxone to preserve life and to mitigate a need for endotracheal intubation and mechanical ventilation, which is an invasive and resource-intensive therapy undertaken by skilled providers.
Our overall goal is identification of an easily administered (e.g., intramuscular or nasal) rescue drug that will provide rapid and prolonged reversal of potent synthetic opioid–induced respiratory depression. In prior work, we had determined that intravenously and intratracheally administered thyrotropin-releasing hormone and its long-acting, serum-stable analog taltirelin were effective in reversing morphine-induced respiratory depression in isoflurane-anesthetized rats (Boghosian et al., 2018). Thyrotropin-releasing hormone is a neurostimulant tripeptide hormone known classically for its role in thyroid hormone homeostasis and its effects in decreasing anesthesia “sleep time” in rodents. In the current project, we tested the hypothesis that taltirelin would provide similar effect in reversing morphine- and sufentanil-induced respiratory depression in restrained, conscious rats as measured by nose-only plethysmograpy and arterial blood gas analysis; sufentanil has a clinical potency and a μ opioid receptor binding affinity ∼1000-fold and ∼10-fold greater than morphine, respectively (Volpe et al., 2011). Unfortunately, taltirelin beneficial effects were limited by worsening metabolic acidosis, perhaps due to increased oxygen consumption. For this reason, we studied in a similar fashion PKTHPP, a potassium channel antagonist (Cotten, 2013, 2016), and additional breathing stimulants identified by others for their effects in reversing sufentanil-induced respiratory depression, including 8-hydroxy-2-(di-n-propylamino)tetralin (8-OH-DPAT), a 5-HT1A and 5-HT7 serotonin receptor agonist (Sahibzada et al., 2000; Meyer et al., 2006); CX717, an ampakine glutamate receptor positive modulator (Ren et al., 2009); BIMU8, a 5-HT4(a) receptor agonist (Manzke et al., 2003); and A85380, an α4β2 nicotinic acetylcholine receptor agonist (Ren et al., 2019). We also studied the effect of prazosin coadministration, an α-1 adrenergic receptor antagonist known to reverse opioid-induced skeletal muscle rigidity (Tsou et al., 1989).
Methods
Animal Studies
All studies were approved by the Massachusetts General Hospital Institutional Animal Care and Use Committee and used 69 male Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA) weighing 303 to 550 g, with none excluded. Femoral artery catheters tunneled and exteriorized through a dorsal incision were implanted by the vendor. Animals were housed in the Massachusetts General Hospital Center for Comparative Medicine.
All experiments were performed on animals restrained in a custom-built full-body acrylic chamber using a size-appropriate Allay neck collar (Supplemental Fig. 1). Animals were acclimated to restraint on two separate sessions of at least 30 minutes each on 2 days prior to study and again for 30 minutes on the study day after recovery from anesthesia and just prior to baseline breathing and arterial blood gas measurements. Unless noted, all study compounds were administered intravenously through a 24G lateral tail vein angiocatheter placed under brief (∼3–5 minutes) inhaled isoflurane anesthesia (3%–5%). An automated heat lamp was used to maintain isoflurane-anesthetized rat body temperature at 37°C via a rectal thermistor. Because conscious animals become agitated by the rectal thermistor and expel it, we did not measure body temperature in conscious rats and allowed them to autoregulate their body temperature.
Breathing Studies
For nose-only breathing studies, the restrained rat's nose and mouth, facilitated by the collar restraint, were positioned in an acrylic “nose chamber” 2.5 × 5 × 5 cm (internal dimension) via a 0.7-cm diameter laser-cut aperture in a latex diaphragm (Supplemental Fig. 1; Hygenic Dental Dam, Benco Dental, Pittston, PA). The nose chamber was continuously flushed with fresh air (1 l/min) via two Luer ports using a mass flow controller (model GE50A with Type 247 power supply; MKS Instruments Inc., Andover, MA). When used, isoflurane was administered by directing airflow upstream of the nose chamber through a variable bypass vaporizer. Rat produced carbon dioxide in the exiting gas flow and isoflurane concentration within the nose chamber, which were measured using a Capstar-100 (CWE, Inc.; Ardmore, PA) and a Capnomac Ultima (GE Healthcare, Buckinghamshire, U.K.), respectively, that were both calibrated daily using a calibration gas mixture (Part 20731570-001; GE Healthcare). Gas flow exiting the nose chamber was quantified using a heated pneumotachometer (model 8420; Hans Rudolph Inc., Shawnee, KS) and a differential pressure transducer and demodulator (models MP45-14871 and CD15; Validyne Engineering, Northridge, CA). The flow and carbon dioxide analog signals were acquired (128 Hz, 4-second time epochs) and analyzed using LabView 2014 software (National Instruments, Austin, TX) run on an Apple computer (Cupertino, CA) interfaced with three USB-6009 data acquisition boards (National Instruments). Oscillations in the gas flow exiting the nose chamber, as imparted by rat breathing, were used to measure breathing frequency and minute ventilation, as detailed elsewhere (Boghosian et al., 2018). Minute ventilation was estimated by numerically integrating the gas flow signal after digital subtraction of the baseline 1 l/min gas flow. The system was calibrated each day by occluding the nose chamber latex diaphragm aperture and by attaching a rodent ventilator (model 683; Harvard Apparatus; Holliston, MA) providing a known minute ventilation (150 ml/min) through a Luer port into the nose chamber. Tidal volume within each 4-second time epoch was calculated by dividing the measured minute ventilation by the frequency.
Arterial Blood Gas Studies
For talitirelin, arterial blood gas analysis and nose-only plethysmography breathing studies were conducted in separate sessions. However, for other study compounds, 8-OH-DPAT, prazosin, BIMU8, A85380, and CX717, arterial blood gas samples were collected during (i.e., simultaneous with) nose-only plethysmography breathing studies. The arterial catheter was accessed through an opening in the plexiglass restraint (Supplemental Fig. 1). Arterial blood samples (approximately 0.3 ml, heparinized at ∼3 units/ml after withdrawal) taken at baseline and at several time points after study compound administrations were analyzed immediately using a Vetscan iStat 1 (Abaxis, Union City, CA) blood gas analyzer and CG4+ cartridges (Abbott Laboratories, Princeton, NJ).
Loss of Righting Reflex Studies
After tail vein catheterization and 30 minutes anesthesia recovery time, sufentanil was administered intravenously, a stopwatch was started, and the rat was placed supine with all four paws “in the air.” Five minutes after sufentanil injection, 8-OH-DPAT or saline (total volume 1 ml/kg) was administered intramuscularly into the right gastrocnemius muscle. When the animal righted with all four paws “on the ground,” the stopwatch was stopped, and the time was recorded.
Drugs and Study Compounds (See Also Supplemental Fig. 2)
Taltirelin (MedChem Express; Monmouth Junction, NJ), 8-OH-DPAT (Sigma-Aldrich, St. Louis, MO), prazosin (Sigma-Aldrich), BIMU8 (Santa Cruz Biotechnology, Inc.; Dallas, TX), A85380 (R&D systems; Minneapolis, MN), and naloxone (Sigma-Aldrich) were all solubilized in sterile 0.9% saline prior to administration. CX717 (custom synthesized by Aberjona Laboratories; Woburn, MA) was solubilized in 10% 2-hydroxypropyl-β-cyclodextrin (Sigma-Aldrich) in sterile 0.9% saline. PKTHPP (custom synthesized by Aberjona Laboratories; Woburn, MA) was solubilized in DMSO. Isoflurane was purchased from Patterson Veterinary (Greeley, CO), and morphine and sufentanil were from McKesson Medical-Surgical (San Francisco, CA). Excluding PKTHPP, all study drugs/compounds were administered in a final total volume of 1 ml. In PKTHPP studies, all animals received 1 ml/kg of DMSO total, which we know from prior work to be well tolerated. All study compounds were flushed into the vein using 0.5 ml of sterile 0.9% saline. For each breathing stimulant compound, we used a higher or a slightly higher dose than that used in published studies involving opioid-induced respiratory depression (Table 1).
TABLE 1.
List of references demonstrating breathing stimulant efficacy in rats and used to guide dosing in the current study.
| Drug | Reference | Dose/Route | Opioid | Anesthesia |
|---|---|---|---|---|
| Taltirelin | Boghosian et al | 1 mg/kg IV | Morphine 5 mg/kg IV over 5 min | Isoflurane |
| PKTHPP | Cotten et al. 2016 | 2.5 mg/kg/hour IV after 0.25 mg/kg IV | Morphine 5 mg/kg IV over 5 min | Isoflurane |
| CX717 | Ren et al. 2009 | 15 mg/kg IV | Fentanyl 80 mcg/kgIV over 1 min | None |
| BIMU8 | Manzke et al. | 1 to 2 mg/kg IV | Fentanyl 10 to 15 mcg/kg IV over 20 min | Pentobarbital |
| A85383 | Ren et al. 2019 | 10 or 30 mcg/kg IV | Fentanyl 60 mcg/kg IV over 20 min | None |
| 8-OH-DPAT | Sahibzada et al. | 10 or 100 mcg/kg IV | Morphine 21 mg/kg IV | Urethane/α-chloralose |
| 8-OH-DPAT | Guenther et al. 2009 | 0.1 to 100 mcg/kg IV | Morphine 13 mg/kg IV | Sevoflurane |
Statistical Analysis
All data are presented as mean ± S.E.M. Statistical analysis was performed using Prism 8.0 for Mac OS X software (GraphPad Software, Inc., La Jolla, CA). Comparisons were conducted using one-way ANOVA analysis followed by Sidak’s multiple comparisons post-test. P values less than 0.05 indicate statistical significance.
Results
Taltirelin Effects on Morphine- and Sufentanil-Induced Respiratory Depression
After collecting 15 minutes of baseline breathing data, rats were injected intravenously with opioid, and after 5 minutes, they were injected with taltirelin or vehicle; breathing data were collected for an additional 40 minutes (Figs. 1 and 2). Morphine (10 mg/kg i.v.; Fig. 1) and sufentanil (10 μg/kg i.v.; Fig. 2) caused a maximum reduction in minute ventilation to 47 ± 4% (n = 7) and 15 ± 4% (n = 6) of baseline, respectively. Sufentanil immediately upon administration caused a period of apnea (∼4 to 8 seconds) followed by a brief period of shallow, irregular breathing (Supplemental Fig. 1). Taltirelin (1 mg/kg i.v.) restored minute ventilation in both morphine- and sufentanil-treated rats by 45 minutes, primarily through effects on breathing rates up to 166 ± 14% of baseline at 54 minutes (n = 8) and up to 163 ± 10% at 54 minutes (n = 6), respectively. Rats coadministered opioid and taltirelin displayed tail quivering and, on occasion, profuse oral secretions, although these effects were not quantified. By arterial blood gas analysis (Fig. 3), morphine and sufentanil both caused respiratory acidosis (i.e., decreased arterial pH and increased carbon dioxide pressure; Fig. 3, A and B) and hypoxia (i.e., a decreased arterial oxygen pressure; Fig. 3C). Taltirelin treatment decreased arterial carbon dioxide pressure but only by 45 minutes after administration and failed to correct hypoxia. Additionally, in morphine- and sufentanil-treated rats, taltirelin caused a significant lactic metabolic acidosis, as indicated by increases in arterial lactate and decreases in base excess relative to opioid-only–treated rats (Fig. 3, D and E).
Fig. 1.
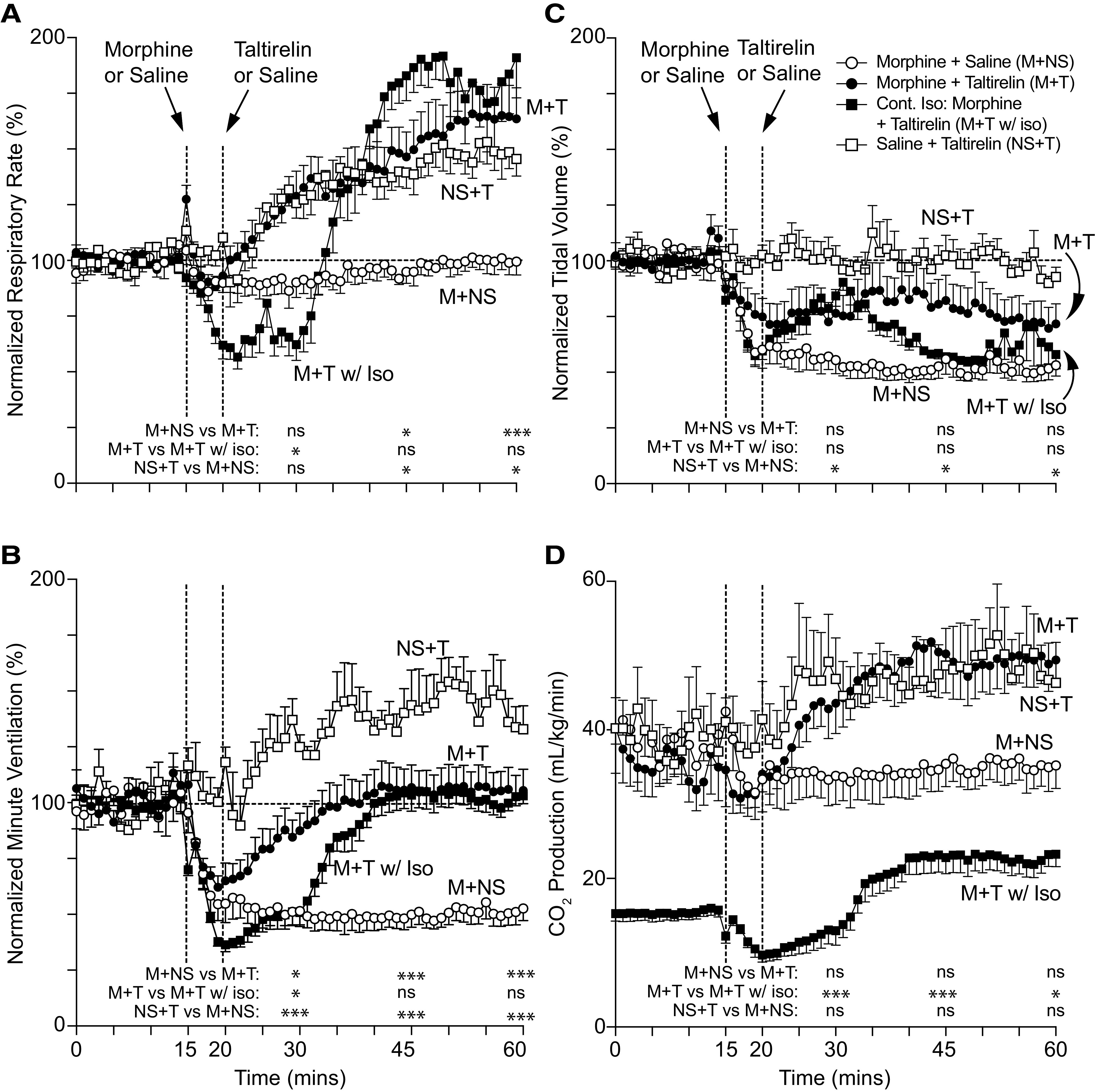
Rat breathing and CO2 production after morphine and taltirelin treatment with and without isoflurane anesthesia. Average respiratory rate (A), minute ventilation (B), tidal volume (C), and exhaled CO2 (D) as measured by nose-only plethysmography vs. time. Animals received 10 mg/kg i.v. morphine (M) over 5 minutes or 1 ml i.v. saline (NS) at 15 minutes followed by 1 mg/kg i.v. taltirelin (T) or 1 ml i.v. saline (NS) at 20 minutes; only one group received anesthesia with continuous 1.5% inhaled isoflurane (w/Iso) throughout. For each animal, data were normalized in (A) through (C) to 15 minutes of baseline data; each data point represents an average of 1 minute of data ± S.E.M.; n = 6–8 animals in (A) through (C) and n = 5–6 in (D). “ns” indicates no significance (P > 0.05); asterisks (∗ and ∗∗∗) indicate statistical significance (P < 0.05, P < 0.001, respectively) by one-way ANOVA test with a Sidak’s multiple comparisons post-test at data points 15, 30, or 45 minutes after first morphine or saline administration. For all conscious experiments, average respiratory rate (rate), minute ventilation (MV), tidal volume (TV), and CO2 production at baseline were 121 ± 4 breaths/min, 53 ± 3 ml/min/100 g, 0.44 ± 0.02 ml/100 g, and 42.6 ± 7.4 ml/kg/min, respectively; n = 21; n = 16 (CO2 production). For isoflurane-anesthetized animals, average respiratory rate, minute ventilation, tidal volume, and CO2 production at baseline were 71 ± 3 breaths/min, 33 ± 1 ml/min/100 g, 0.46 ± 0.02 ml/100 g, and 15.4 ± 0.9 ml/kg/min, respectively; n = 6.
Fig. 2.
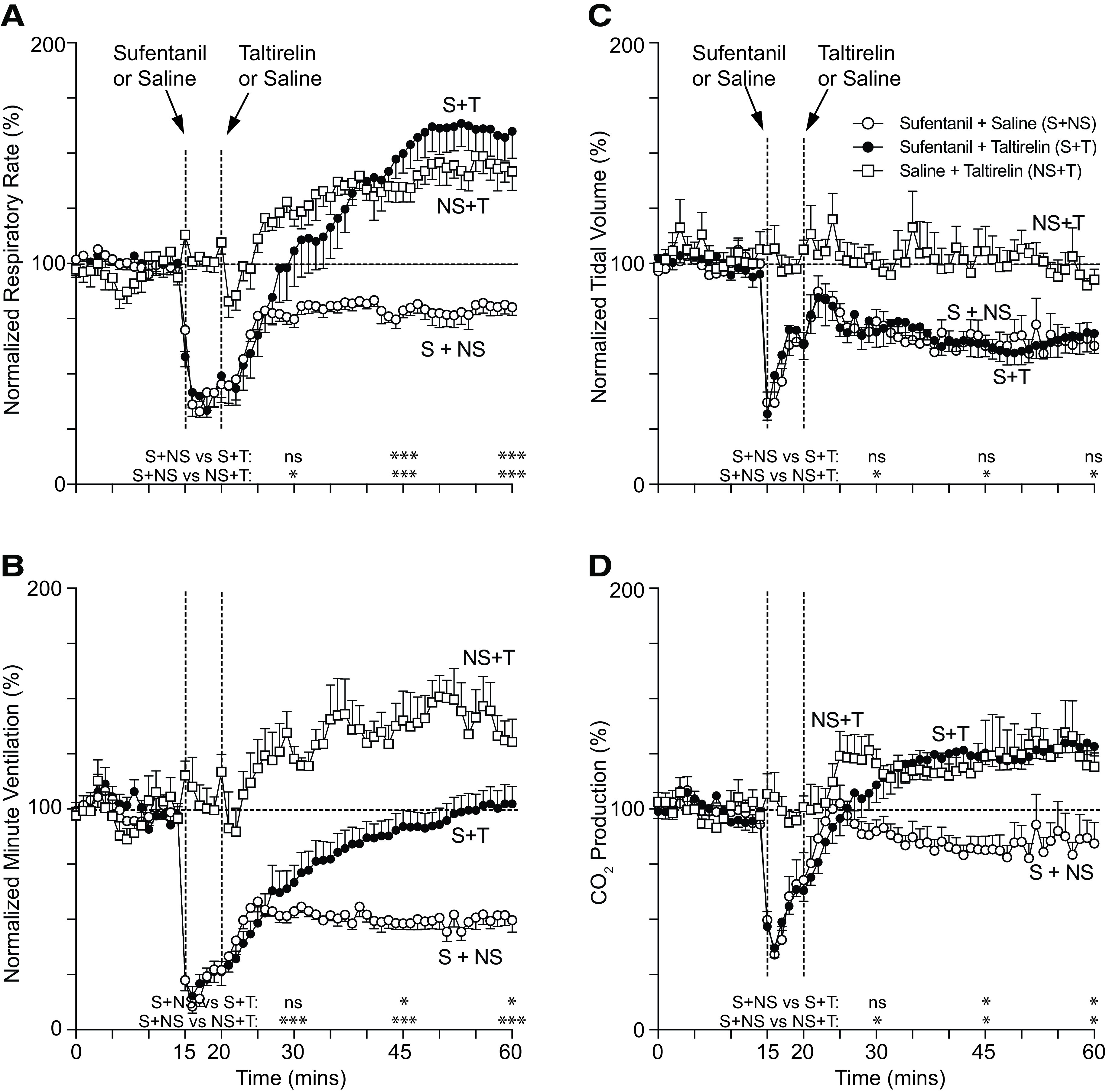
Conscious rat breathing and CO2 production after sufentanil and taltirelin treatment. Avg. respiratory rate (A), minute ventilation (B), tidal volume (C), and exhaled CO2 (D) as measured by nose-only plethysmography vs. time. Animals received 10 μg/kg i.v. sufentanil (S) by bolus (over ∼5-10 seconds) or 1 ml i.v. saline (NS) at 15 minutes followed by 1 mg/kg i.v. taltirelin (T) or 1 ml i.v. saline (NS) at 20 minutes. Data were averaged and normalized similar to Fig. 1 and were collected from n = 6 animals. “ns” indicates no significance (P > 0.05); asterisks (∗ and ∗∗∗) indicate statistical significance (P < 0.05, P < 0.0001, respectively) by one-way ANOVA test with Sidak’s multiple comparisons post-test at data points 15, 30, or 45 minutes after first sufentanil or saline administration. Baseline avg. respiratory rate, minute ventilation, tidal volume, and CO2 production at baseline were 126 ± 3 breaths/min, 55 ± 2 ml/min/100 g, 0.44 ± 0.02 ml/100 g, and 33.0 ± 1.8 ml/kg/min, respectively; n = 18.
Fig. 3.
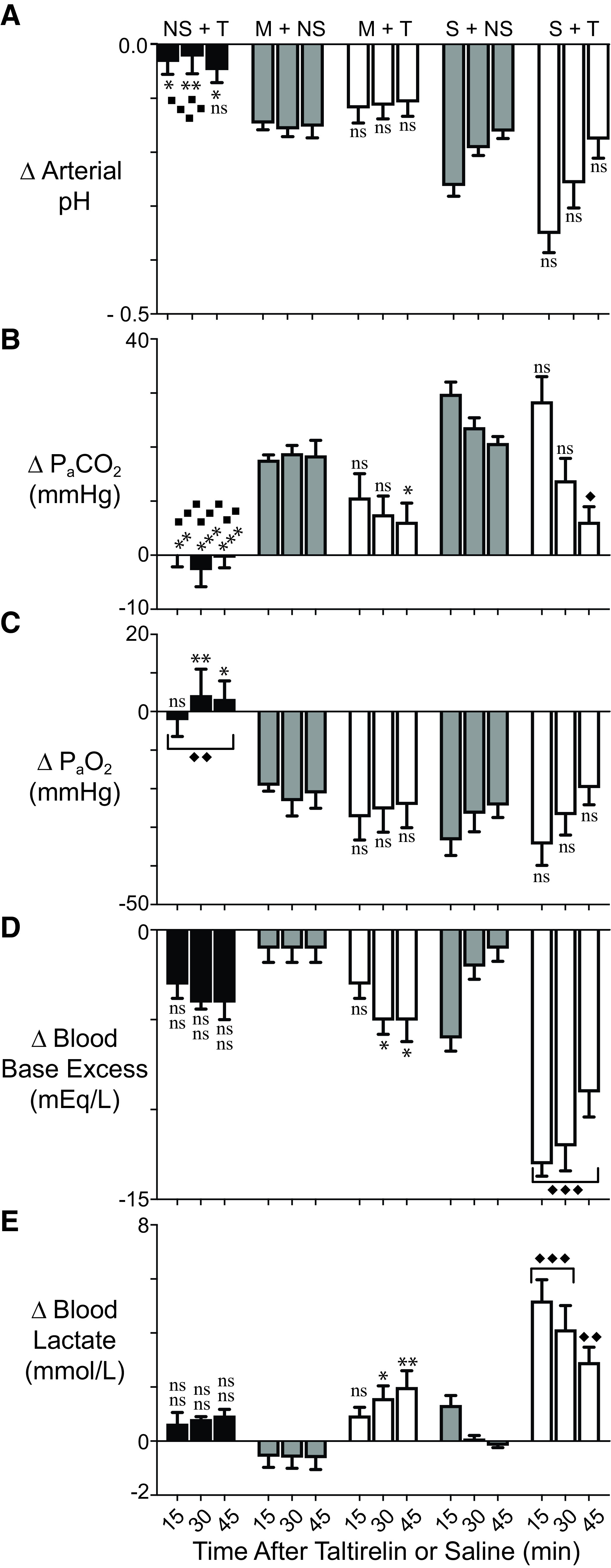
Morphine, sufentanil, and taltirelin effects on arterial blood pH (A), carbon dioxide (B), and oxygen (C) partial pressures and base excess (D) and lactate (E) levels in conscious, air-breathing rats. Data were collected using animals different than those of Figs. 1 and 2. Drug dosing and administration order and times were as in Figs. 1 and 2: 10 mg/kg i.v. morphine (M) (over 5 minutes) or 10 μg/kg i.v. sufentanil (S) by bolus (over ∼5-10 seconds) or 1 ml i.v. saline (NS) at 15 minutes followed by 1 mg/kg i.v. taltirelin (T) or 1 ml i.v. saline (NS) at 20 minutes. Data indicate change (Δ) in measured value from baseline—collected just prior to first opioid/saline administration—and 15, 30, and 45 minutes after taltirelin (or saline) administration. Data are avg. from 6–8 animals (n = 6–8). “ns” indicates no significance (P > 0.05); asterisks and diamonds (∗/⧫, ∗∗/⧫⧫, and ∗∗∗/⧫⧫⧫) indicate statistical significance (P < 0.05, P < 0.01, and P < 0.001) by one-way ANOVA test with a Sidak’s multiple comparisons post-test relative to morphine plus saline (M+NS) or sufentanil plus saline (S+NS) animals, respectively. Baseline arterial blood pH, PaCO2, PaO2, base excess, and lactate were 7.48 ± 0.01, 38.9 ± 0.8 mmHg, 90 ± 2 mmHg, +5.0 ± 0.3 mEq/L, and 0.7 ± 0.1 mmol/L, respectively; n = 21.
In prior studies of isoflurane-anesthetized, morphine-treated rats—and in contrast to our current results in conscious rats (Fig. 3) —taltirelin caused normalization of both arterial oxygen and carbon dioxide pressure levels within 15 minutes of administration (Boghosian et al., 2018). We hypothesized that isoflurane and taltirelin effects on oxygen consumption might contribute to this discrepancy. We therefore measured exhaled, steady-state carbon dioxide levels as an estimate of oxygen consumption in both conscious and isoflurane-anesthetized, morphine-treated rats. We used morphine because sufentanil bolus administration to isoflurane-anesthetized rats is consistently lethal. Isoflurane, despite maintenance of body temperature at 37°C, caused a significant decrease in basal carbon dioxide production by greater than 65% from 42.6 ± 7.4 to 15.4 ± 0.9 ml/kg/min, respectively (Fig. 1D; n = 16 and 6, respectively). Relative to opioid-only–treated rats, taltirelin administration increased carbon dioxide production by up to 64% (Figs. 1D and 2D).
PKTHPP, 8-OH-DPAT, CX717, BIMU8, and A85380 Effects on Sufentanil-Induced Respiratory Depression
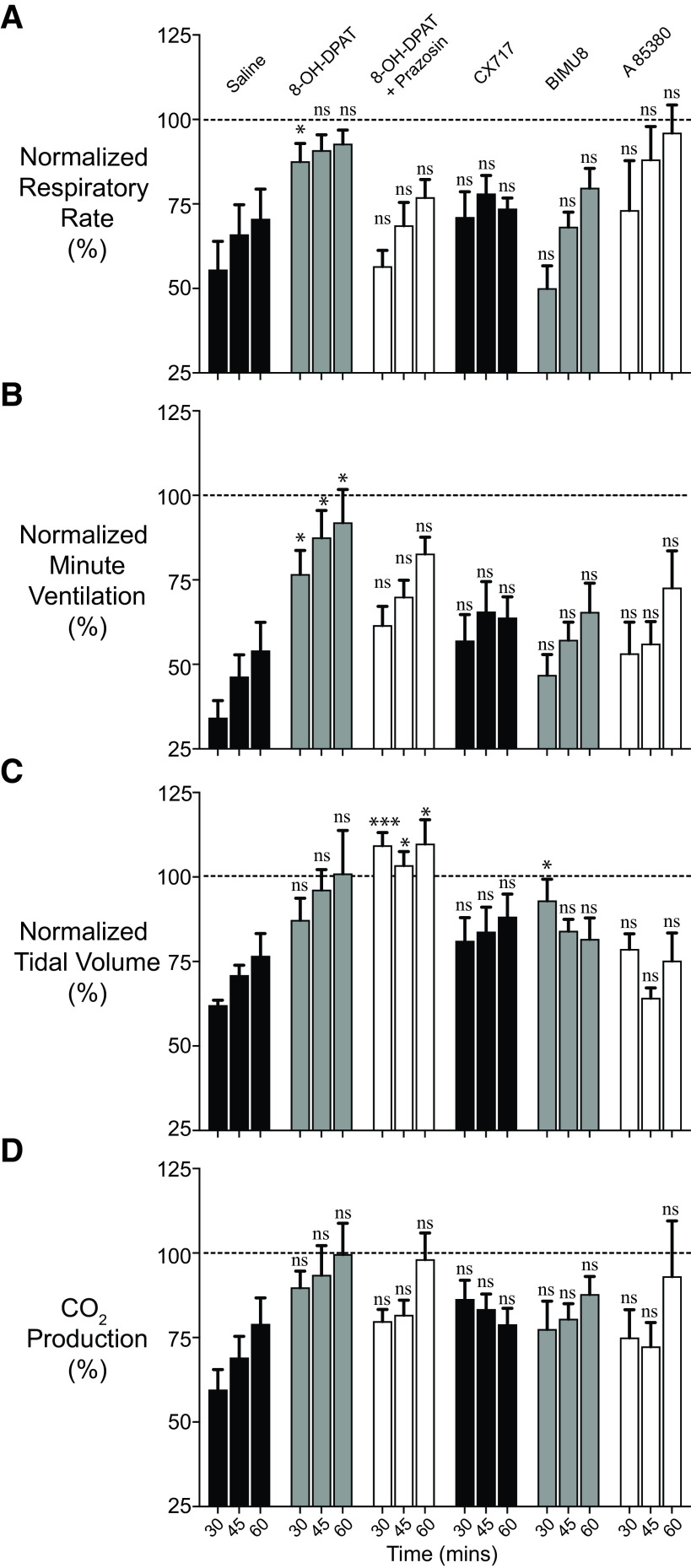
Because opioid-plus-taltirelin–treated rats faired poorly both subjectively (i.e., quivering tail and profuse oral secretions) and objectively (i.e., persistent hypoxia and metabolic acidosis), we studied and compared the effects of additional breathing stimulants in reversing sufentanil-induced respiratory depression. 8-OH-DPAT improved minute ventilation, breathing rate, pH, and PaCO2 (Figs. 4–6). Coadministration of prazosin, an antihypertensive with muscle-relaxant properties, with 8-OH-DPAT improved tidal volume (at the expense of minute ventilation and rate) (Fig. 5), which led to improved oxygenation (Fig. 6C). However, other than an increase in tidal volume at one time point for BIMU8, none of the agents, including PKTHPP, CX717, BIMU8, and A85380, caused significant changes in breathing or arterial blood gas and chemistry measurements (Figs. 5 and 6; Supplemental Figs. 3–6).
Fig. 4.
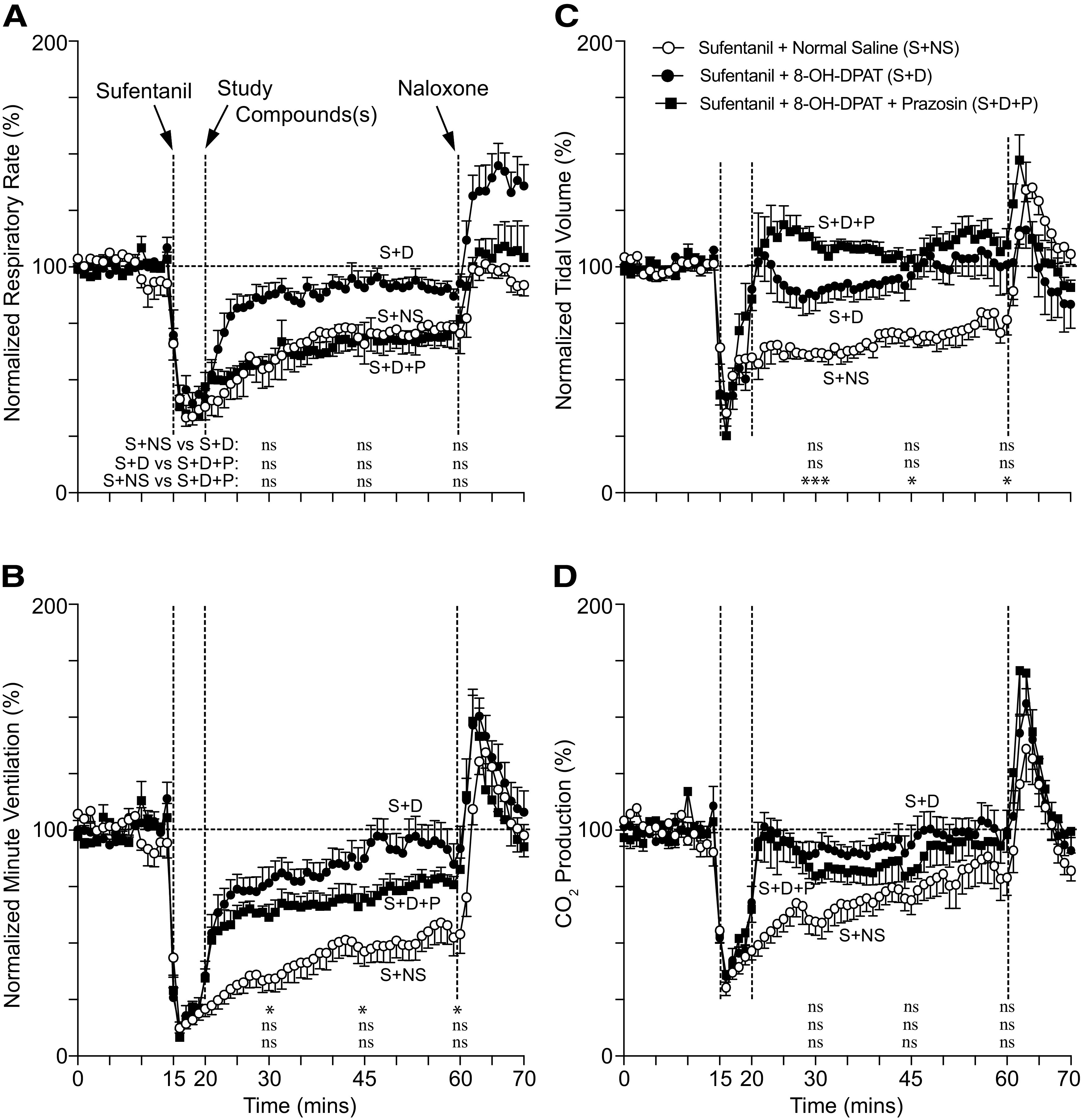
Conscious rat breathing and CO2 production after sufentanil, 8-OH-DPAT, and prazosin treatment. Avg. respiratory rate (A), minute ventilation (B), tidal volume (C), and exhaled CO2 (D) as measured by nose-only plethysmography vs. time. Animals received 10 μg/kg i.v. sufentanil (S) by bolus (over ∼5 to 10 seconds) at 15 minutes followed by 150 μg/kg i.v. 8-OH-DPAT (D) or 150 μg/kg i.v. 8-OH-DPAT plus 250 μg/kg i.v. prazosin (P) at 20 minutes and 1 mg/kg i.v. naloxone at 60 minutes. Data were averaged and normalized similar to Fig. 1 and were collected from n = 6 animals. “ns” indicates no significance (P > 0.05); asterisks (∗ and ∗∗∗) indicate statistical significance (P < 0.05, P < 0.001, respectively) by one-way ANOVA test with Sidak’s multiple comparisons post-test at data points 15, 30, or 45 minutes after sufentanil. Baseline avg. respiratory rate, minute ventilation, tidal volume, and CO2 production for all experiments were 127 ± 4 breaths/min, 59 ± 2 ml/min/100 g, 0.47 ± 0.02 ml/100 g, and 29.2 ± 1.7 ml/kg/min, respectively; n = 18.
Fig. 6.
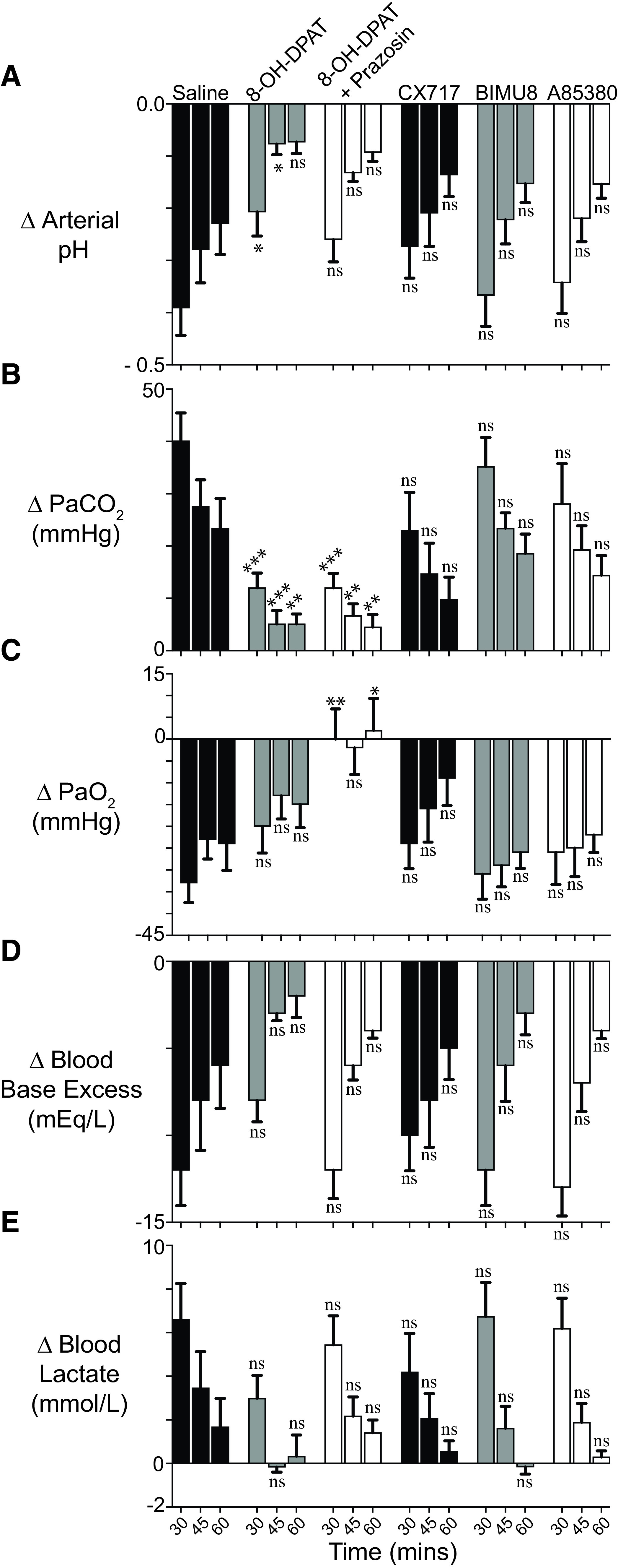
Sufentanil, 8-OH-DPAT, prazosin, CX717, BIMU8, and A85380 effects on arterial blood pH (A), carbon dioxide (B), and oxygen (C) partial pressures and base excess (D) and lactate (E) levels in conscious, air-breathing rats. Blood gas data were collected on the same animals simultaneously with the breathing data of Figs. 4 and 5, such that drug dosing and administration order and times are the same. Data indicate change (Δ) in measured value from baseline—collected just prior to first opioid administration—and 15, 30, and 45 minutes after study drug(s) (or saline) administration. Data are avg. from six animals (n = 6). “ns” indicates no significance (P > 0.05); asterisks and diamonds (∗, ∗∗, and ∗∗∗) indicate statistical significance (P < 0.05, P < 0.01, and P < 0.001) by one-way ANOVA test with a Sidak’s multiple comparisons post-test relative to sufentanil plus saline (S+NS) animals, respectively, at the same time point. Baseline arterial blood pH, PaCO2, PaO2, base excess, and lactate were 7.44 ± 0.01, 41.0 ± 0.6 mm Hg, 90 ± 1 mm Hg, +3.0 ± 0.3 mEq/L, and 1.38 ± 0.1 mmol/L, respectively; n = 36.
Fig. 5.
Conscious rat breathing and CO2 production after sufentanil, 8-OH-DPAT, prazosin, CX717, BIMU8, and A85380 treatment. Avg. respiratory rate (A), minute ventilation (B), tidal volume (C), and exhaled CO2 (D) as measured by nose-only plethysmography at three time points (30, 45, and 60 minutes); this includes 8-OH-DPAT data shown in Fig. 4. Rats received 10 μg/kg i.v. sufentanil at 15 minutes followed by 1) 1 ml of saline, 2) 150 μg/kg i.v. 8-OH-DPAT, 3) 150 μg/kg i.v. 8-OH-DPAT plus 250 μg/kg i.v. prazosin, 4) 30 mg/kg i.v. CX717, 5) 30 μg/kg i.v. A85380, or 6) 1 mg/kg i.v. BIMU8 at 20 minutes. Data were averaged and normalized similar to Fig. 1 and were collected from n = 6 animals for each compound. “ns” indicates no significance (P > 0.05); asterisks (∗ and ∗∗∗) indicate statistical significance (P < 0.05, P < 0.001, respectively) by one-way ANOVA test with Sidak’s multiple comparisons post-test at data points. The avg. baseline respiratory rate, minute ventilation, tidal volume, and CO2 production for all experiments were 128 breaths/min, 61 ± 1 ml/min/100 g, 0.47 ± 0.01 ml/100 g, and 29.8 ± 1.0 ml/kg/min, respectively; n = 36.
Intramuscular 8-OH-DPAT Effects on Sufentanil-Induced Respiratory Depression, Sedation, and Immobility
Because bioavailability and route of administration are important considerations in a rescue agent, we studied 8-OH-DPAT when administered by intramuscular injection. Intramuscular 8-OH-DPAT caused normalization of minute ventilation within 10 minutes of administration (Fig. 7) to sufentanil-treated rats. 8-OH-DPAT did increase exhaled carbon dioxide, which will need to be explored in future studies (Fig. 7D).
Fig. 7.
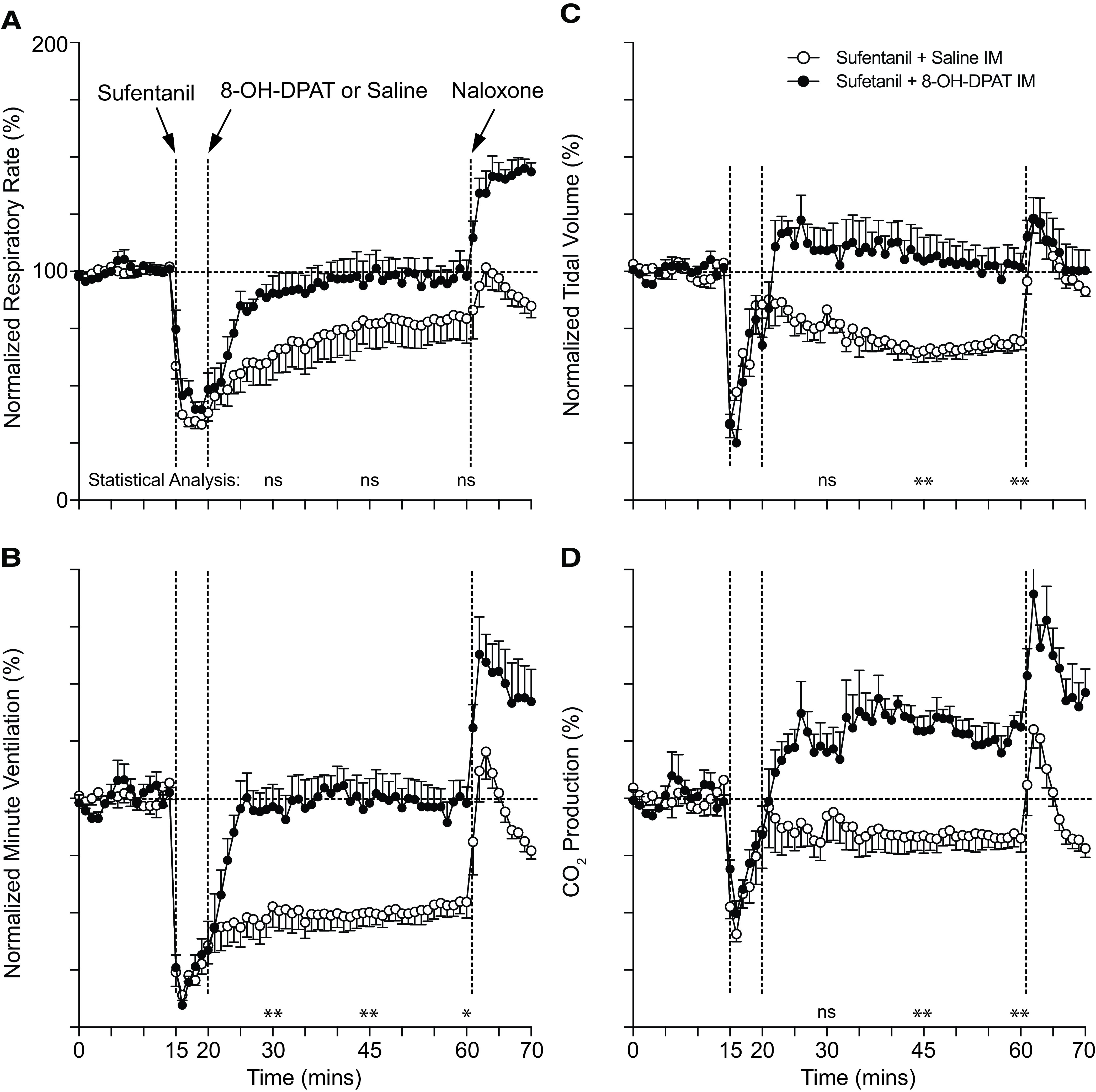
Conscious rat breathing and CO2 production after intravenous sufentanil and intramuscular 8-OH-DPAT treatment. Avg. respiratory rate (A), minute ventilation (B), tidal volume (C), and exhaled CO2 (D) as measured by nose-only plethysmography vs. time. Animals received 10 μg/kg i.v. sufentanil (S) by bolus (over ∼5 to 10 seconds) at 15 minutes followed by 150 μg/kg i.m. 8-OH-DPAT (D) or 1 ml/kg i.m. normal saline at 20 minutes and 1 mg/kg i.v. naloxone at 60 minutes. Data were averaged and normalized similar to Fig. 1 and were collected from n = 6 animals. “ns” indicates no significance (P > 0.05); asterisks (∗ and ∗∗) indicate statistical significance (P < 0.05, P < 0.01, respectively) by one-way ANOVA test with Sidak’s multiple comparisons post-test at data points 15, 30, or 45 minutes after sufentanil. Baseline avg. respiratory rate, minute ventilation, tidal volume, and CO2 production for all experiments were 120 ± 3 breaths/min, 58 ± 1 ml/min/100 g, 0.48 ± 0.01 ml/100 g, and 28.9 ± 1.2 ml/kg/min, respectively; n = 24.
To determine whether 8-OH-DPAT reverses sufentanil-induced sedation and immobility, we placed rats supine immediately after sufentanil (10 μg/kg i.v.) administration. Intramuscular injection with 8-OH-DPAT shortened time to righting from 43 ± 4 to 15 ± 1 minutes (n = 8 and 9; P < 0.001; unpaired Student's t test). Sufentanil-saline–injected rats had a slow, continuous transition to righting, sometimes laying on their side for a period; sufentanil-8-OH-DPAT–injected rats, in contrast, tended to flip abruptly from supine to prone shortly after first signs of behavioral arousal. After righting, sufentanil-saline–injected rats remained immobile, whereas sufentanil-8-OH-DPAT–injected rats commenced exploring, despite some jerkiness in their movement.
Discussion
We compared the effects of several breathing stimulants in reversing sufentanil-induced respiratory depression in conscious rats. We studied taltirelin, PKTHPP, CX717, BIMU8, A85380, and 8-OH-DPAT using plethysmography and arterial blood analysis. Although taltirelin stimulated breathing, its effects were offset by metabolic acidosis, possibly from increased oxygen consumption. PKTHPP, CX717, BIMU8, and A85380 failed to provide significant breathing stimulation; however, 8-OH-DPAT did, and coadministration of prazosin improved tidal volume and oxygenation. Intramuscular 8-OH-DPAT was effective in breathing restoration and decreased time to righting in sufentanil-treated rats.
The taltirelin results were informative. We had previously determined that taltirelin 1 mg/kg i.v. fully corrected the hypoxia and hypercarbia caused by 5 mg/kg i.v. morphine in isoflurane-anesthetized rats (Boghosian et al., 2018). In conscious rats, taltirelin worsened morphine-induced hypoxia and partially corrected hypercarbia despite ventilation normalization (Figs. 1 and 3). We used a higher morphine dose (10 mg/kg i.v.) in the current study to provide an equivalent level of respiratory depression (∼50%). As others have observed (Li et al., 2012), isoflurane caused a decrease (>65%) in steady-state carbon dioxide production (Figs. 1 and 3), an estimate of oxygen consumption, which may preserve oxygenation and lessen hypercarbia. Anesthesia also prevented muscle rigidity, tail quivering, and sometimes profuse oral secretions. Finally, taltirelin caused a decrease in arterial blood base excess and an increase in lactate levels in sufentanil-treated rats (Fig. 3). Lactic metabolic acidosis implies increased anaerobic metabolism from oxygen supply-demand mismatch. Taltirelin stimulates oxygen consumption (Puissant et al., 2015) and increases carbon dioxide production.
Other contributors to metabolic acidosis might be inadequate oxygen uptake, delivery, and impaired utilization. Consideration of oxygen consumption is very important in evaluating the therapeutic potential of a breathing stimulant and has not been previously reported upon in the context of opioid reversal; any drug/compound that enhances oxygen consumption will stimulate breathing but may have less therapeutic potential, as it could promote hypoxia, hypercarbia, and acidosis. Therefore, our taltirelin observations identified novel, important, and frequently ignored considerations in breathing stimulant evaluation: 1) presence of anesthesia, 2) stimulant effects on breathing as well as arterial blood gas and chemistry, and 3) stimulant effects on oxygen consumption.
We studied stimulants with differing pharmacologic targets and that were previously shown to be effective in reversing opioid-induced respiratory depression (Table 1) using similar dosing. No one has previously published a study directly comparing multiple stimulants, which is essential in assessing their relative translational potential and in determining which warrant further investigation (e.g., large animal studies). Of these, 8-OH-DPAT was the only to increase minute ventilation and to decrease arterial carbon dioxide pressures relative to sufentanil-only–treated rats. The other stimulants, however, did trend toward improved ventilation and decreased arterial carbon dioxide. The negative results for some may be due to 1) opioid type, dose, and administration rate and 2) lack of anesthesia. A high opioid dose with rapid administration likely causes a greater degree of respiratory depression, rigidity, hypoxia, and acidemia. This is illustrated by arterial blood gas and chemistry results from rats treated with an equipotent morphine and sufentanil dose (Fig. 3). Finally, some prior studies of stimulants were conducted using anesthetized animals (Table 1). Although general anesthetics do worsen respiratory depression, they decrease muscle rigidity, oral secretions (our subjective impression), and oxygen consumption. Therefore, anesthetics may facilitate stimulant effects, as we observed with taltirelin (Figs. 1 and 2) (Boghosian et al., 2018).
Prior studies have demonstrated 8-OH-DPAT reverses or prevents opioid-induced respiratory depression in rats and goats (Table 1) (Sahibzada et al., 2000; Meyer et al., 2006; Dutschmann et al., 2009; Guenther et al., 2009). Because 8-OH-DPAT showed the greatest effects in our study and because it provides beneficial effects in a larger mammal, the etorphine-treated goat, we also undertook studies to address its potential as a first-responder rescue agent. By plethysmography, intramuscular 8-OH-DPAT administration was as effective as intravenous administration in reversing sufentanil-induced respiratory depression (Fig. 7); notably, we did not undertake arterial blood gas and chemistry analysis of rats treated intramuscularly. 8-OH-DPAT is one of several 5-HT1A Gαi protein–coupled receptor agonist compounds [e.g., buspirone, befiridol (NLX-112/F13640), NLX-101 (F15599), and repinotan (BAYx3702)]. 8-OH-DPAT is also a low-affinity 5-HT7 agonist, but its breathing effects are through 5-HT1A, as demonstrated by 5-HT1A antagonist studies (Sahibzada et al., 2000; Guenther et al., 2009). Other selective 5-HT1A agonists are effective in reversing opioid-induced respiratory depression, including bifiridol (NLX-112/F13640) in fentanyl-treated, conscious rats (Ren et al., 2015) and repinotan in remifentanil- or morphine-treated, sevoflurane-anesthetized rats (Guenther et al., 2010; Guenther et al., 2012). Buspirone, a partial 5-HT1A agonist, was effective in anesthetized rats (Sahibzada et al., 2000) but given orally failed to prevent morphine-induced respiratory depression in humans (Oertel et al., 2007). Bifiridol (NLX-112) and NLX-101 are in development by Neurolixis (San Diego, CA) for treatment of l-DOPA–induced dyskinesia, a Parkinson disease treatment side effect, and for depressive disorders and for breathing difficulties in Rett syndrome, respectively. Repinotan was studied in humans as a therapy for stroke (Teal et al., 2009) and brain injury (Ohman et al., 2001). There are two general 5-HT1A receptor populations, presynaptic and postsynaptic. Since 5-HT1A receptors are Gαi protein–coupled and cause neuronal inhibition through potassium channel activation, presynaptic 5-HT1A receptor activation in brainstem raphe neurons may cause breathing stimulation by disinhibition (Sahibzada et al., 2000; Oertel et al., 2007). This hypothesis can be tested since agonists “biased” for presynaptic (F13714) and postsynaptic (NLX-101) 5-HT1A receptors are available. Finally, 8-OH-DPAT may provide toxicity, as “fatal circulatory failure and pulmonary edema” were observed in morphine-treated, sevoflurane-anesthetized rats (Guenther et al., 2009) at the highest dose (100 μg/kg). We observed no overt toxicity in rats using 150 μg/kg, and none was reported in Sahibzada et al. (2000) and Cheng et al. (2016) at doses up to 1 mg/kg.
Opioids cause skeletal muscle rigidity in rodents (Barnett et al., 1975) and humans (Streisand et al., 1993), and this may cause acute death associated with fentanyl overdose (Torralva and Janowsky, 2019). In rodents, rigidity causes the “Straub phenomenon,” which includes a rigid, S-shaped, dorsiflexed tail and extension paralysis of the hind limbs (Bilbey et al., 1960). A rigid “wooden chest” and laryngospasm (Bennett et al., 1997) may compromise mask ventilation in patients receiving high-dose fentanyl-based anesthesia for cardiac surgery. A noncompliant chest wall or airway limits tidal volume to impede ventilation and promote hypoxia. Oxygenation effects are important since hypoxia, as caused by hypoventilation, is what causes death. Skeletal muscle rigidity can be quantified by electromyogram activity, mechanography, or subjective means and is diminished by coadministration of α1-adrenergic antagonists (e.g., prazosin) (Lui et al., 1990), α2-adrenergic agonists (e.g., dexmedetomidine) (Jerussi et al., 1987; Weinger et al., 1989), benzodiazepines (e.g., diazepam) (Sanford et al., 1994), and general anesthetics (Jerussi et al., 1987). Also, 8-OH-DPAT (300 and 1000 μg/kg i.p.) itself “abolishes” fentanyl-induced rigidity in rats (Jaros and Kolasiewicz, 1995). We observed that prazosin coadministration with 8-OH-DPAT improved both tidal volume and oxygenation (Figs. 4–6), suggesting that rigidity eradication is a worthy approach to augment stimulant effects. This novel finding suggests that opioid-induced rigidity is a “road block” to breathing stimulation and adds a new approach and new considerations in developing and testing pharmacologic strategies to reverse opioid-induced respiratory depression. We, however, cannot exclude that prazosin oxygenation effects may be mediated by other mechanism(s), such as improved lung ventilation–blood perfusion matching. Muscle-relaxant agents, such as prazosin, may impose additional toxicities (e.g., hypotension, sedation, or further respiratory depression), which must be considered. Finally, we determined that intramuscular 8-OH-DPAT caused a 64% decrease in time to righting in sufentanil-treated rats. The righting effects imply that 8-OH-DPAT reverses both sedation and skeletal muscle rigidity, which is consistent with the study by Jaros and Kolasiewicz (1995). We speculate that 8-OH-DPAT effects in reversing opioid-induced respiratory depression may be due in part to muscle relaxation. These novel and important findings highlight the potential utility of 8-OH-DPAT as a rescue agent that, after intramuscular administration, can reverse opioid-induced respiratory depression as well as sedation and immobility.
There are limitations to our studies. First, we studied a single dose of each drug, both opioid and stimulant. Although the synthetic opioid lethal dose in conscious rats is high (Janssen, 1982), we chose a dose that provides reliable respiratory depression and a practical recovery time. In future work, more extreme opioid doses should be studied. For the stimulants, we chose the highest or a slightly higher dose than that used in published studies (Table 1). Therefore, this was not an exhaustive comparison between stimulants. Second, we did not study hemodynamic effects (e.g., cardiac rhythm, rate, and blood pressure), an important factor in opioid recovery. Third, rats may be a less-than-ideal animal model, as they are resistant to opioid-induced respiratory depression relative to nonhuman primates, which better mimic the human opioid response. However, nonhuman primates and other large mammals are costly and for ethical and technical reasons are impractical to study and best reserved for validation work. Rats, given their size, cost, and wealth of published data, are a more reasonable model for preclinical discovery work. Finally, nose-only plethysmography requires restraint, which might be a stressor and provide an exaggerated response to sufentanil depressant effects and its subsequent reversal. However, after two acclimatization sessions before first study, rats are more restraint-tolerant. Relative to unrestrained, whole-body plethysmography, nose-only studies provide 1) more precise and dynamic inhaled gas composition measurement and control, 2) tidal volume measurements independent of chamber and body temperature and chamber humidity estimates, 3) simplified arterial blood removal and venous drug administration, 4) simplified exhaled CO2 measurement, and 5) less artifact (e.g., sniffing or exploring).
Potent synthetic opioids are an important cause of overdose death in the ongoing opioid health crisis and are a chemical threat to both the public and the military. In our survey of several breathing stimulants as potential therapeutics, 8-OH-DPAT was the most effective and without overt toxicity. Futures studies employing 8-OH-DPAT and other 5-HT1A agonists are warranted and might address 1) magnitude and duration of breathing effects with larger sufentanil doses and with other potent synthetic opioids (e.g., carfentanil); 2) hemodynamic effects; 3) additional routes of administration (e.g., nasal or inhaled/intratracheal); 4) effects on opioid-induced skeletal muscle rigidity, immobility, and discoordination; 5) breathing enhancement by muscle-relaxant coadministration (e.g., with a α2-adrenergic agonist, such as dexmedetomidine); and 6) coadministration studies with naloxone. Such studies will delineate the therapeutic potential of this compound class and clarify which, if any, warrant further development.
Abbreviations
- 8-OH-DPAT
8-hydroxy-2-(di-n-propylamino)tetralin; mEq, milliequivalent
Authorship Contributions
Participated in research design: Dandrea, Cotten
Conducted experiments: Dandrea
Contributed new reagents or analytic tools: Dandrea, Cotten
Performed data analysis: Dandrea, Cotten
Wrote or contributed to the writing of the manuscript: Dandrea, Cotten
Footnotes
This research was supported by the Massachusetts General Hospital Department of Anesthesia, Critical Care & Pain Medicine and by Countermeasures Against Chemical Threats (CounterACT) Program supplemental awards to National Institutes of Health National Institute on Drug Abuse [Grant R21-DA045231].
No author has an actual or perceived conflict of interest with the contents of this article.
 This article has supplemental material available at jpet.aspetjournals.org.
This article has supplemental material available at jpet.aspetjournals.org.
References
- Barnett A, Goldstein J, Fiedler E, Taber R (1975) Etonitazine-induced rigidity and its antagonism by centrally acting muscle relaxants. Eur J Pharmacol 30:23–28. [DOI] [PubMed] [Google Scholar]
- Bennett JA, Abrams JT, Van Riper DF, Horrow JC (1997) Difficult or impossible ventilation after sufentanil-induced anesthesia is caused primarily by vocal cord closure. Anesthesiology 87:1070–1074. [DOI] [PubMed] [Google Scholar]
- Bilbey DL, Salem H, Grossman MH (1960) The anatomical basis of the straub phenomenon. Br J Pharmacol Chemother 15:540–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boghosian JD, Luethy A, Cotten JF (2018) Intravenous and Intratracheal Thyrotropin Releasing Hormone and Its Analog Taltirelin Reverse Opioid-Induced Respiratory Depression in Isoflurane Anesthetized Rats. J Pharmacol Exp Ther 366:105–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng JP, Leary JB, Sembhi A, Edwards CM, Bondi CO, Kline AE (2016) 5-hydroxytryptamine1A (5-HT1A) receptor agonists: A decade of empirical evidence supports their use as an efficacious therapeutic strategy for brain trauma. Brain Res 1640 (Pt A):5–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotten JF (2013) TASK-1 (KCNK3) and TASK-3 (KCNK9) tandem pore potassium channel antagonists stimulate breathing in isoflurane-anesthetized rats. Anesth Analg 116:810–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotten JF, Boghosian JD, Luethy A(2016) PKTHPP Enhances the Ventilatory Response to Carbon Dioxide and Reverses Opioid Induced Respiratory Depression, in American Society of Anesthesiologists, Annual Meeting of Chicago, Illinois. [Google Scholar]
- Dahan A, Aarts L, Smith TW (2010) Incidence, Reversal, and Prevention of Opioid-induced Respiratory Depression. Anesthesiology 112:226–238. [DOI] [PubMed] [Google Scholar]
- Doucette C(2017) Carfentanil: The drug of mass destruction, in Toronto Sun [Google Scholar]
- Dutschmann M, Waki H, Manzke T, Simms AE, Pickering AE, Richter DW, Paton JF (2009) The potency of different serotonergic agonists in counteracting opioid evoked cardiorespiratory disturbances. Philos Trans R Soc Lond B Biol Sci 364:2611–2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guenther U, Manzke T, Wrigge H, Dutschmann M, Zinserling J, Putensen C, Hoeft A (2009) The counteraction of opioid-induced ventilatory depression by the serotonin 1A-agonist 8-OH-DPAT does not antagonize antinociception in rats in situ and in vivo. Anesth Analg 108:1169–1176. [DOI] [PubMed] [Google Scholar]
- Guenther U, Theuerkauf NU, Huse D, Boettcher MF, Wensing G, Putensen C, Hoeft A (2012) Selective 5-HT(1A)-R-agonist repinotan prevents remifentanil-induced ventilatory depression and prolongs antinociception. Anesthesiology 116:56–64. [DOI] [PubMed] [Google Scholar]
- Guenther U, Wrigge H, Theuerkauf N, Boettcher MF, Wensing G, Zinserling J, Putensen C, Hoeft A (2010) Repinotan, a selective 5-HT1A-R-agonist, antagonizes morphine-induced ventilatory depression in anesthetized rats. Anesth Analg 111:901–907. [DOI] [PubMed] [Google Scholar]
- Janssen PA (1982) Potent, new analgesics, tailor-made for different purposes. Acta Anaesthesiol Scand 26:262–268. [DOI] [PubMed] [Google Scholar]
- Jaros T, Kolasiewicz W (1995) Attenuation of the fentanyl-induced muscle rigidity by the selective 5HT1A agonist 8-OH-DPAT. Pol J Pharmacol 47:19–24. [PubMed] [Google Scholar]
- Jerussi TP, Capacchione JF, Benvenga MJ (1987) Reversal of opioid-induced muscular rigidity in rats: evidence for alpha-2 adrenergic involvement. Pharmacol Biochem Behav 28:283–289. [DOI] [PubMed] [Google Scholar]
- Li RQ, McKinstry AR, Moore JT, Caltagarone BM, Eckenhoff MF, Eckenhoff RG, Kelz MB (2012) Is hydrogen sulfide-induced suspended animation general anesthesia? J Pharmacol Exp Ther 341:735–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lui PW, Lee TY, Chan SH (1990) Involvement of coerulospinal noradrenergic pathway in fentanyl-induced muscular rigidity in rats. Neurosci Lett 108:183–188. [DOI] [PubMed] [Google Scholar]
- Manzke T, Guenther U, Ponimaskin EG, Haller M, Dutschmann M, Schwarzacher S, Richter DW (2003) 5-HT4(a) receptors avert opioid-induced breathing depression without loss of analgesia. Science 301:226–229. [DOI] [PubMed] [Google Scholar]
- Meyer LC, Fuller A, Mitchell D (2006) Zacopride and 8-OH-DPAT reverse opioid-induced respiratory depression and hypoxia but not catatonic immobilization in goats. Am J Physiol Regul Integr Comp Physiol 290:R405–R413. [DOI] [PubMed] [Google Scholar]
- Oertel BG, Schneider A, Rohrbacher M, Schmidt H, Tegeder I, Geisslinger G, Lötsch J (2007) The partial 5-hydroxytryptamine1A receptor agonist buspirone does not antagonize morphine-induced respiratory depression in humans. Clin Pharmacol Ther 81:59–68. [DOI] [PubMed] [Google Scholar]
- Ohman J, Braakman R, Legout V; Traumatic Brain Injury Study Group (2001) Repinotan (BAY x 3702): a 5HT1A agonist in traumatically brain injured patients. J Neurotrauma 18:1313–1321. [DOI] [PubMed] [Google Scholar]
- Puissant MMEchert AEYang CMouradian GC Jr, Novotny T, Liu P, Liang MHodges MR (2015) RNASeq-derived transcriptome comparisons reveal neuromodulatory deficiency in the CO2 insensitive brown Norway rat. J Physiol 593:415–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren J, Ding X, Funk GD, Greer JJ (2009) Ampakine CX717 protects against fentanyl-induced respiratory depression and lethal apnea in rats. Anesthesiology 110:1364–1370. [DOI] [PubMed] [Google Scholar]
- Ren J, Ding X, Greer JJ (2015) 5-HT1A receptor agonist Befiradol reduces fentanyl-induced respiratory depression, analgesia, and sedation in rats. Anesthesiology 122:424–434. [DOI] [PubMed] [Google Scholar]
- Ren J, Ding X, Greer JJ (2019) Activating α4β2 Nicotinic Acetylcholine Receptors Alleviates Fentanyl-induced Respiratory Depression in Rats. Anesthesiology 130:1017–1031. [DOI] [PubMed] [Google Scholar]
- Sahibzada N, Ferreira M, Wasserman AM, Taveira-DaSilva AM, Gillis RA (2000) Reversal of morphine-induced apnea in the anesthetized rat by drugs that activate 5-hydroxytryptamine(1A) receptors. J Pharmacol Exp Ther 292:704–713. [PubMed] [Google Scholar]
- Sanford TJ Jr, Weinger MB, Smith NT, Benthuysen JL, Head N, Silver HBlasco TA (1994) Pretreatment with sedative-hypnotics, but not with nondepolarizing muscle relaxants, attenuates alfentanil-induced muscle rigidity. J Clin Anesth 6:473–480. [DOI] [PubMed] [Google Scholar]
- Schiermeier Q (2002) Hostage deaths put gas weapons in spotlight. Nature 420:7. [DOI] [PubMed] [Google Scholar]
- Shafer SL (2019) Carfentanil: a weapon of mass destruction. Can J Anaesth 66:351–355. [DOI] [PubMed] [Google Scholar]
- Streisand JB, Bailey PL, LeMaire L, Ashburn MA, Tarver SD, Varvel J, Stanley TH (1993) Fentanyl-induced rigidity and unconsciousness in human volunteers. Incidence, duration, and plasma concentrations. Anesthesiology 78:629–634. [DOI] [PubMed] [Google Scholar]
- Sutter ME, Gerona RR, Davis MT, Roche BM, Colby DK, Chenoweth JA, Adams AJ, Owen KP, Ford JB, Black HB, et al. (2017) Fatal Fentanyl: One Pill Can Kill. Acad Emerg Med 24:106–113. [DOI] [PubMed] [Google Scholar]
- Teal P, Davis S, Hacke W, Kaste M, Lyden PD, Fierus M; Modified Randomized Exposure Controlled Trial Study Investigators; Bayer HealthCare AG (2009) A randomized, double-blind, placebo-controlled trial to evaluate the efficacy, safety, tolerability, and pharmacokinetic/pharmacodynamic effects of a targeted exposure of intravenous repinotan in patients with acute ischemic stroke: modified Randomized Exposure Controlled Trial (mRECT). Stroke 40:3518–3525. [DOI] [PubMed] [Google Scholar]
- Torralva R, Janowsky A (2019) Noradrenergic Mechanisms in Fentanyl-Mediated Rapid Death Explain Failure of Naloxone in the Opioid Crisis. J Pharmacol Exp Ther 371:453–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsou MY, Lui PW, Lee TY, Pan JT, Chan SH (1989) Differential effects of prazosin and yohimbine on fentanyl-induced muscular rigidity in rats. Neuropharmacology 28:1163–1168. [DOI] [PubMed] [Google Scholar]
- Uddayasankar U, Lee C, Oleschuk C, Eschun G, Ariano RE (2018) The Pharmacokinetics and Pharmacodynamics of Carfentanil After Recreational Exposure: A Case Report. Pharmacotherapy 38:e41–e45. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Koob GF, McLellan AT (2016) Neurobiologic Advances from the Brain Disease Model of Addiction. N Engl J Med 374:363–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpe DA, McMahon Tobin GA, Mellon RD, Katki AG, Parker RJ, Colatsky T, Kropp TJ, Verbois SL (2011) Uniform assessment and ranking of opioid μ receptor binding constants for selected opioid drugs. Regul Toxicol Pharmacol 59:385–390. [DOI] [PubMed] [Google Scholar]
- Weinger MB, Segal IS, Maze M (1989) Dexmedetomidine, acting through central alpha-2 adrenoceptors, prevents opiate-induced muscle rigidity in the rat. Anesthesiology 71:242–249. [DOI] [PubMed] [Google Scholar]
- Wilson N, Kariisa M, Seth P, Smith H 4th, Davis NL (2020) Drug and Opioid-Involved Overdose Deaths - United States, 2017-2018. MMWR Morb Mortal Wkly Rep 69:290–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung DT, Bough KJ, Harper JR, Platoff GE Jr (2020) National Institutes of Health (NIH) Executive Meeting Summary: Developing Medical Countermeasures to Rescue Opioid-Induced Respiratory Depression (a Trans-Agency Scientific Meeting)-August 6/7, 2019. J Med Toxicol 16:87–105. [DOI] [PMC free article] [PubMed] [Google Scholar]