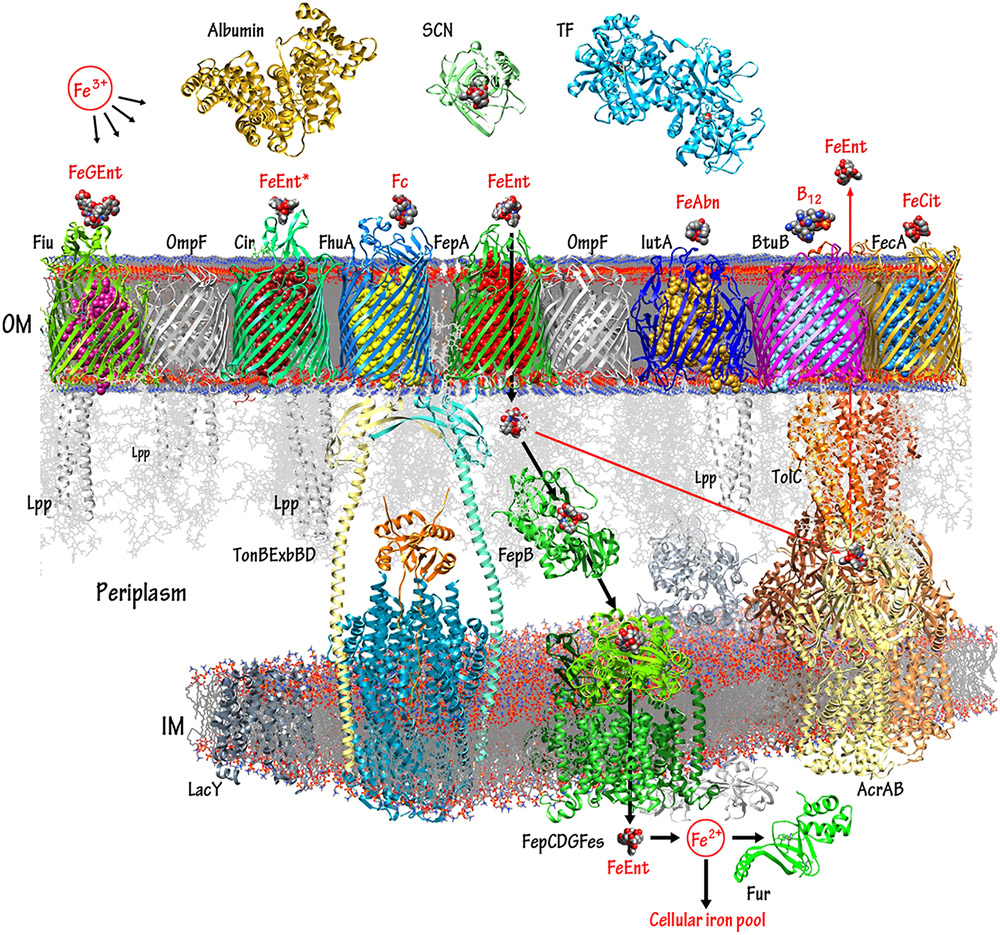
Figure 1.
TonB-dependent iron and B12 transport pathways in Gram (−) bacteria. The diagram displays selected components of the E. coli OM, periplasm, and IM, rendered by CHIMERA (UCSF) from their RCSB crystallographic coordinates. Proteins that participate in metal flux are portrayed in colors; other cell envelope components are shown in shades of gray. Bacteria and fungi secrete siderophores that chelate extracellular iron. In human and animal hosts, the innate immune system proteins albumin, SCN, and TF antagonize bacterial iron acquisition, by adsorbing siderophores, ferric siderophores, or free iron from blood, serum, lymph, and other fluids. Nevertheless, high affinity bacterial OM LGP bind specific ferric siderophores (or vitamin B12) and actively transport them into the periplasm. The bacterial TonB/ExbBD complex spans the cell envelope and utilizes IM PMF to energize the OM active transport reactions.91,237 TonB/ExbBD is modeled from the crystallographic coordinates of the TonB C-terminus,241 the ExbBD proteins,232,233,707 and other data;236,644,708 the full complex was not yet structurally delineated. The import (black arrows) and export (red arrows) pathways of FeEnt typify those of other metal complexes: after binding and TonB-dependent internalization by FepA, FeEnt binds to the periplasmic protein FepB that delivers it to the IM ABC-transporter FepCDG, which hydrolyzes ATP as it transports the ferric siderophore to the cytoplasm. During or after the IM uptake process, Fes hydrolyzes the lactone backbone of FeEnt, which effectively releases Fe3+ for reduction to Fe2+.709 Ferrous iron enters cellular iron pools, and equilibrium with the global regulator, Fur.710-712 Alternatively, if surplus FeEnt exists in the periplasm, then the AcrABTolC export complex expels the excess to the exterior.62 The depiction of FepCDGFes was modeled from the crystal structure of BtuCD.