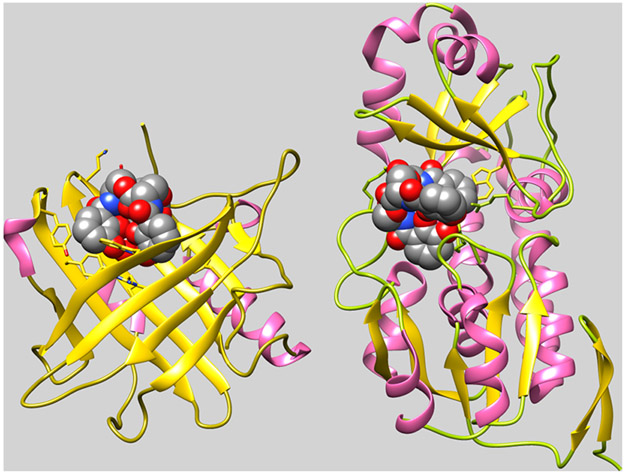
Figure 2.
Binding of FeEnt by HsaSCN and EcoFepB. Comparison of the crystallographic structures of human SCN (3CMP) and E. coli FepB (3TLK), with bound FeEnt, shows two different structural folds for FeEnt binding. Both contain α- (pink) and β- (gold) structures, but the former human serum protein binds FeEnt in the mouth of a seven-stranded β-barrel, whereas the latter periplasmic protein binds it in the central cleft of a bilobed globule. In both cases, however, affinity for the aromatic, triply negatively charged ferric siderophore derives from interactions with cationic (SCN: R81, R130, R134; FepB: R78, R242, R301) and aromatic (SCN: Y52, W79, Y100, Y106, F123, Y132; FepB: F300, W209) side chains in the binding protein. Adsorption of FeEnt to EcoFepA involves similar contributions of charge713 and aromaticity714,715 to the overall affinity.