FIG. 7.
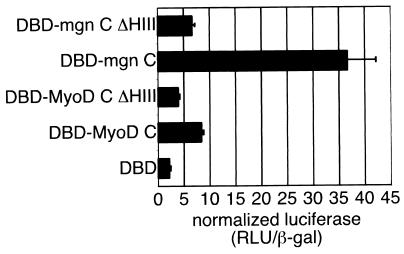
Myogenin possess a helix III-dependent C-terminal activation domain. The C termini of MyoD and myogenin were assayed for the ability to function as a general activation domain when fused to the Gal4 DBD (amino acids 1 to 147). Gal fusion proteins were generated by PCR amplifying cDNA encoding MyoD amino acids 170 to 318 (DBD-MyoD C) and myogenin amino acids 136 to 224 (DBD-mgn C) and ligating the PCR products into the pSG424 vector. Similar fusion proteins with deletions of the helix III motif in MyoD (amino acids 245 to 258; DBD-MyoD C ΔHIII) and myogenin (amino acids 195 to 208; DBD-mgn C ΔHIII) were also made. The Gal4-C terminus chimeras were cotransfected into NIH 3T3 cells with a Gal4-responsive luciferase reporter plasmid and a constitutively expressed β-galactosidase reporter to normalize for transfection efficiency. Each transfection was repeated three times. The graph represents the mean normalized luciferase activity (relative light units [RLU]/β-galactosidase activity) for the three experiments. Error bars represent standard deviations.