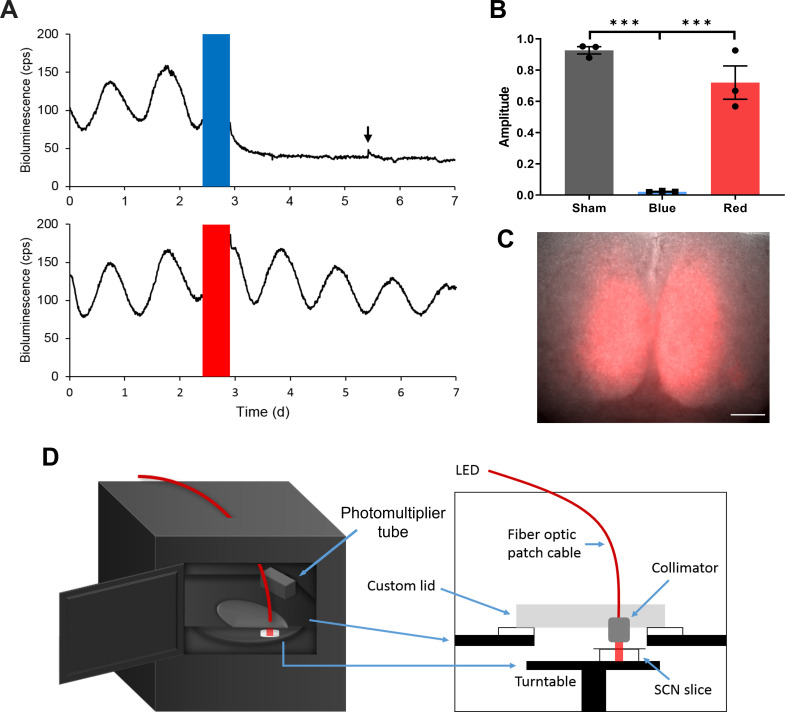
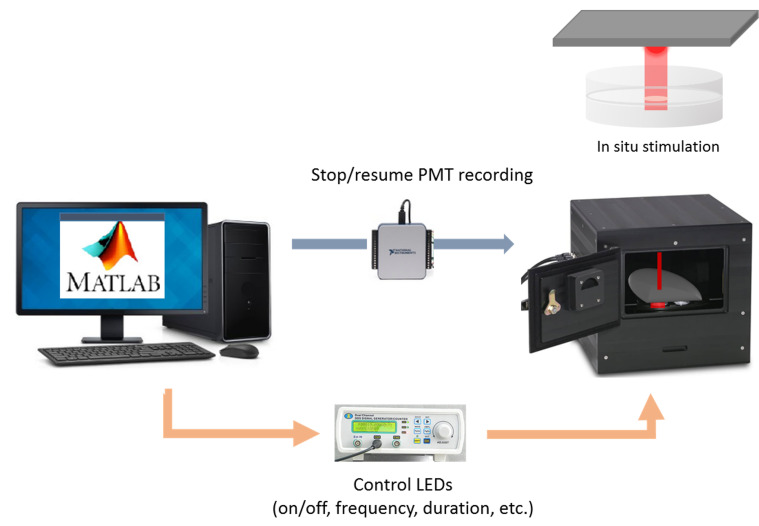
Figure 1. Long-term Optogenetic Stimulation System for Circadian Entrainment Ex Vivo.
(A) Representative PER2::LUC bioluminescence rhythms of adult SCN slices exposed to either red (top) or blue (bottom) 10 Hz light pulses (red or blue bars) for 12 hr. The black arrow indicates the timing of media change. (B) Fold change in the rhythm amplitude following sham, blue, or red light exposure (Student’s t-test, mean ± SEM, n = 3, ***p < 0.001). (C) Merged ChrimsonR-tdT fluorescence and the brightfield images of an SCN slice. Scale = 100 μm. (D) Diagrams showing a multi-channel luminometer integrated with an optogenetic stimulation apparatus.