Abstract
Objectives
The first large nosocomial cluster of coronavirus disease 2019 (COVID-19) in Singapore in April 2021 led to partial closure of a major acute care hospital. This study examined factors associated with infection among patients, staff and visitors; investigated the possible role of aerosol-based transmission; evaluated the effectiveness of BNT162.b2 and mRNA1273 vaccines; and described the successful containment of the cluster.
Methods
Close contacts of patients with COVID-19 and the affected ward were identified and underwent surveillance for severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection. Patient, staff and visitor cohorts were constructed and factors associated with infection were evaluated. Phylogenetic analysis of patient samples was performed. Ward air exhaust filters were tested for SARS-CoV-2.
Results
In total, there were 47 cases, comprising 29 patients, nine staff, six visitors and three household contacts. All infections were of the Delta variant. Ventilation studies showed turbulent air flow and swabs from air exhaust filters were positive for SARS-CoV-2. Vaccine breakthrough infections were seen in both patients and staff. Among patients, vaccination was associated with a 79% lower odds of infection with COVID-19 (adjusted odds ratio 0.21, 95% confidence interval 0.05–0.95).
Conclusions
This cluster occurred despite enhancement of infection control measures that the hospital had undertaken at the onset of the COVID-19 pandemic. It was brought under control rapidly through case isolation, extensive contact tracing and quarantine measures, and led to enhanced use of hospital personal protective equipment, introduction of routine rostered testing of inpatients and staff, and changes in hospital infrastructure to improve ventilation within general wards.
Keywords: COVID-19, COVID-19 vaccines, Outbreak, Phylogeny, Infection control
Introduction
Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), has spread rapidly around the world with over 250 million cases reported by 1st November 2021. New variants of concern have also been identified, including the Delta variant [1], which have rapidly replaced the original strain and led to surges in infections globally.
Singapore has registered more than 201,000 cases of COVID-19 and 412 deaths from COVID-19-related complications. Prior to August 2021, Singapore maintained a containment strategy that aimed to detect, isolate and contact trace every case of confirmed COVID-19.
Tan Tock Seng Hospital is a 1600-bed multi-disciplinary acute care hospital in Singapore. Prior to this cluster, visitors to the hospitals as well as outpatients were required to use a face mask (surgical or fabric mask) by law. Admitted patients were encouraged to use face masks if their condition permitted. Entry swabs were performed where there was suggestive clinical [e.g. symptoms of acute respiratory infection (ARI)] or contact history, and visitors with ARI symptoms were restricted from entering the hospital. A multi-pronged approach to protect staff against COVID-19 – which includes routine use of surgical masks for patient interactions; N95 respirators and eye protection, gowns and gloves when performing high-risk aerosol-generating procedures (AGPs) or interacting with known or suspected cases of COVID-19; daily temperature monitoring; and active surveillance for ARI – has also been implemented since January 2020 [2]. With these measures, nosocomial transmission of SARS-CoV-2 did not occur in the hospital until April 2021 [3,4], when the first large nosocomial cluster of COVID-19 in Singapore occurred with the Delta variant.
This study examined factors associated with infection among patients, staff and visitors; investigated the possible role of aerosol-based transmission; evaluated the effectiveness of BNT162.b2 and mRNA1273 vaccines; and described the successful containment of the cluster.
Methods
The outbreak occurred in General Ward I, a 43-bed facility comprising six six-bed cubicles separated by floor-to-ceiling side-walls, a two-bed isolation room, a single-bedroom used for end-of-life patients, one enclave bed, three temporary corridor beds that are used during periods of high hospital occupancy, and common toilets and showers. The ward is equipped with air conditioners that are turned on when indoor ambient temperatures exceed 27 °C.
The index case, a nurse (Patient A) on Ward I, presented with fever on 27th April 2021 and a positive nasopharyngeal swab for SARS-CoV-2 later that night. On 28th April 2021, a symptomatic physician (Patient B) who had seen patients on Ward I tested positive for SARS-CoV-2, as did a patient (Patient C) who had been lodged on Ward I since 20th April 2021. In response, the ward was closed on 28th April 2021. Epidemiological investigations, contact tracing and quarantine of close contacts were performed. In addition, all hospital staff were swabbed and screened weekly, and a thorough review of all existing infection control measures was taken.
Patients who were still on Ward I at the time of closure (28th April 2021) were transferred to isolation rooms, placed on active clinical surveillance, and underwent nasal/throat swabs for SARS-CoV-2 polymerase chain reaction (PCR) tests (every 1–3 days). Discharged patients, visitors to Ward I, and household contacts of COVID-19 cases were issued 14-day quarantine orders by the Ministry of Health Singapore, swabbed on entry to and exit from quarantine, and thereafter required to further isolate at home for another 7 days. Staff were quarantined, and swabbed at days 7, 14 and 21 from the last date of contact with a confirmed case or with Ward I. All PCR tests were performed in accredited laboratories using a nationally approved PCR test protocol.
Close contacts (individuals exposed for ≥15 min within 2 m of a confirmed case of COVID-19), and patients, staff and visitors who spent ≥15 min cumulatively in Ward I from 20th April 2021 (the date that Patient C was admitted to Ward I) to 28th April 2021 (date of ward closure) were identified. This was accomplished through reviews of electronic medical records, staff duty rosters, patient and visitor registration records, closed-circuit television footage, data from a real-time location system [5] that was used in the emergency department (ED), and self-reporting via an internet-based survey platform.
Three cohorts were constructed: (i) patients admitted to Ward I; (ii) visitors to Ward I; and (iii) staff based on Ward I (e.g. nurses, healthcare assistants, housekeepers) and non-ward-based staff (allied health professionals) rostered to Ward I.
The following variables were collected: (i) patients: demographics, co-morbidities, mobility status, receipt of potential aerosol-generating procedures (AGPs) (open suctioning of endotracheal or tracheostomy tubes, nebulizer treatment, sputum induction and high-flow oxygen supplementation), SARS-CoV-2 vaccination status, date of COVID-19 symptom onset, dates and times of admission and discharge from Ward I, and bed location within the ward; (ii) visitors: dates and times of visits to Ward I, and the patient visited; and (iii) healthcare workers: demographics, SARS-CoV-2 vaccination status, duration of exposure in Ward I, and patient(s) on Ward I for whom care was provided.
Standard descriptive statistics of distribution and measures of central tendency were performed. Multiple logistic regression was used to assess factors associated with COVID-19. All reported P-values were two-tailed, with an α level of 0.05. Statistical analyses were conducted using Stata Version 13.1 (StataCorp LLC, College Station, TX, USA).
Whole-genome sequencing of SARS-Co-V-2 samples was performed as described [6]. Insertion/deletion mutations were screened using Freebayes [7], and verified in consensus sequences after examining raw reads. Only sequences that were 98% complete and supported by an average coverage of 100x were shared via GISAID [8] and included. All sequences were aligned using MAFFT v7.427 [9]. The maximum-likelihood tree was inferred using IQ-TREE v2.0.3 with best-fit model, ultrafast bootstrap approximation [[10], [11], [12], [13]] and hCoV-19/Wuhan/WIV04/2019 (accession EPI_ISL_402124) as a reference root. Additionally, the closest available Delta sequences were downloaded from GISAID and added as references.
Air exhaust filters from the air-conditioning system in Ward I were extracted, and the entire surface of each filter (each measuring 970 x 370 x20 mm, made of synthetic fibre) was swabbed with two to three swabs (Eswab; COPAN, Murrieta, CA, USA) and collected in viral transport medium [14]. Material was dislodged by vortexing, and clarified by centrifugation. The supernatant was subjected to RNA purification (MagMAX/Kingfisher; ThermoFisher, Waltham, MA, USA), followed by an N1-specific TaqMan assay performed in triplicate to detect SARS-CoV-2 RNA [15].
Data collection and analyses were performed as part of the hospital's outbreak response, and approved for publication by the Domain Specific Review Board of National Healthcare Group (Ref. No. 2021/00511).
Results
Case finding identified 47 cases in the cluster, comprising 29 patients (28 from Ward I, one from Ward II), nine staff, six visitors to Ward I and three household contacts. While the majority of these 47 cases had mild infections, four deaths (three of which were attributed to COVID-19) occurred in patients who had multiple co-morbidities and had not received SARS-CoV-2 vaccination.
Contact tracing identified a total of 220 patients (admitted to Ward I or II between 20th and 28th April 2021, or from other wards who were close contacts of infected medical staff), 416 staff who were close contacts of cases of COVID-19, 634 other staff who had spent at least 15 min on Ward I or Ward II, and 741 visitors to either ward. In addition, 11,004 asymptomatic hospital staff were screened weekly for SARS-CoV-2 without any covert infection detected [16].
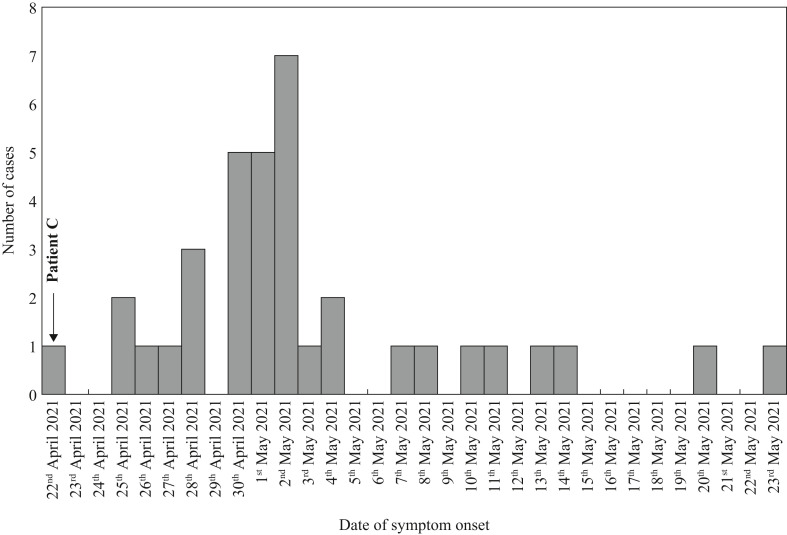
The epidemic curve of 36 cases who were symptomatic (Figure 1 ) is consistent with a point source outbreak, although epidemiological investigations suggest that there were at least three cases of secondary transmission. Patient C was most likely the primary case.
Figure 1.
Epidemic curve observed in the Ward I cluster.
Patient C presented at the ED on 18th April 2021 with a 3-day history of fever, upper respiratory symptoms and headache. A nasopharyngeal swab for SARS-CoV-2 was negative and chest X-ray was normal. The clinical impression was a sinus infection with scalp cellulitis, and the patient was admitted to another ward before being transferred to Ward I on 20th April 2021. He was first admitted to a bed in Cubicle 6 on 20th April 2021, and transferred to Cubicle 1 on 22nd April 2021. The patient's fever resolved in response to antibiotics, but recurred on 22nd April 2021. A computed tomography scan of the abdomen and pelvis to investigate the cause of persistent fever showed ground glass opacities in the lower lung fields. The patient was then isolated, and a second PCR swab, performed on 28th April 2021, was positive for COVID-19.
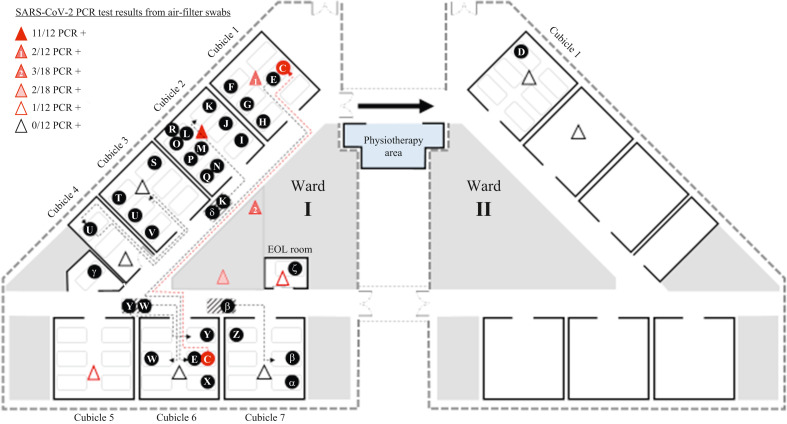
Patient D was admitted to Ward II on 19th April 2021, and remained there until a PCR swab tested positive on 29th April 2021. The patient (unvaccinated) had no contact with any patients with COVID-19 or staff from Ward I. No other patients on Ward II tested positive. Wards I and II are separate wards with independent ventilation systems, but are connected by an internal corridor (Figure 3 ), which leads to a shared physiotherapy area. This area was used by several COVID-19-positive patients and staff from Ward I between 20th and 28th April 2021.
Figure 3.
Bed location of all patient cases of coronavirus disease 2019 in the Ward I cluster, and air exhaust filter swab results. SARS-CoV-2, severe acute respiratory syndrome coronavirus-2; PCR, polymerase chain reaction.
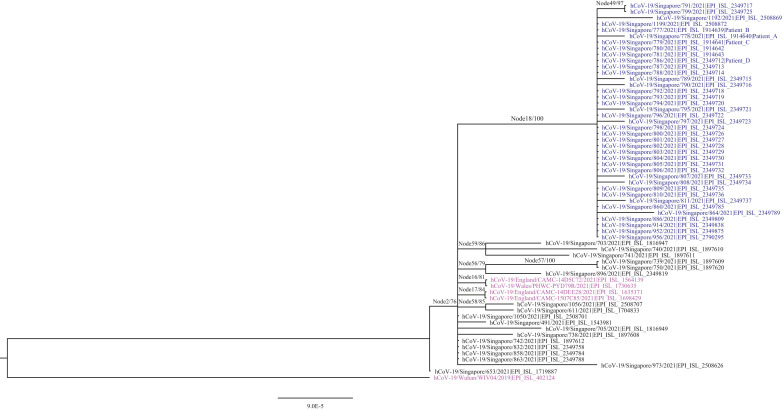
All samples from 39 cases in the Ward I COVID-19 cluster were of the Delta variant, clustering on the same branch with 100% bootstrap validation (Figure 2). The remaining cases could not be sequenced adequately due to low viral load. This supported the epidemiological findings of a single introduction event of SARS-CoV-2 into Ward I. The virus from the patient in neighbouring Ward II and non-ward-based staff also clustered on this branch. No patient, staff or visitor from the first ward that Patient C had been admitted to tested positive for SARS-CoV-2.
Figure 2.
Phylogenetic relationship of severe acute respiratory syndrome coronavirus-2 sequences, as generated by IQTree with γ-distributed rate differences and 1000 bootstrap validation. All sequences were identified by GISAID accession numbers, with global and Singapore references in magenta and black, respectively. Cases related to Ward I are in blue (Node 18). The scale bar indicates genetic distance between sequences, and bootstrap values are indicated at node branches. Samples from Patient A (index case), Patient B (symptomatic physician), Patient C (primary case) and Patient D (patient from Ward II) are shown.
The cubicle with the largest number of COVID-19 infections in this cluster (Cubicle 2) was not either of the cubicles to which the primary case was admitted. No patients in Cubicle 5 acquired COVID-19, although this cubicle was fully occupied throughout the risk period (Figure 3).
Air filters from various locations in the ward were extracted approximately 9 days after the ward was closed, swabbed and tested for SARS-CoV-2. Air filters from Cubicles 1 and 2 and the nursing station were positive for SARS-CoV-2, with Cubicle 2 having the greatest proportion of swabs that were positive (Figure 3). All swabs from air filters in Cubicles 1 (location of Patient D) and 2 of Ward II were negative.
Of the 92 patients in Ward I between 20th and 28th April 2021, the attack rate was 30.4% (28 infected). The mean length of stay on the ward was significantly longer for patients with COVID-19 than non-cases (5.6 ± 2.7 days vs 3.0 ± 2.7 days; P<0.001). The adjusted odds of infection increased with duration of exposure on the ward [adjusted odds ratio (OR) 1.38, 95% confidence interval (CI) 1.14–1.68].
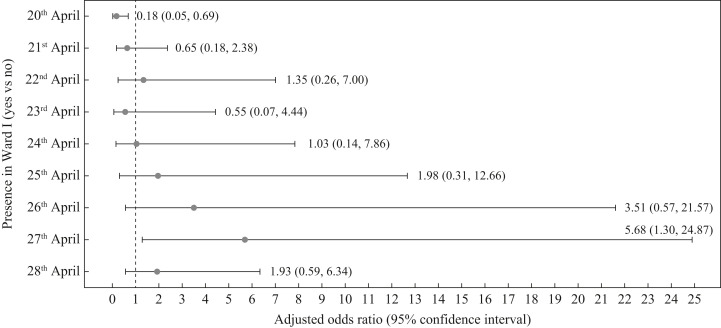
The odds of infection increased with ward stay in the later part of the period 20th–28th April, and was estimated to be 5.68 times higher for ward stay on 27th April (95% CI 1.30–24.87) compared with not staying on the ward that day (Figure 4 ). Patients in Cubicle 2 (adjusted OR 23.98, 95% CI 3.44–167.01) and in corridor beds (adjusted OR 9.56, 95% CI 1.49–61.15) had higher odds of acquiring COVID-19.
Figure 4.
Adjusted odds ratio for stay in Ward I on specific days during the period from 20th to 28th April 2021. Note: Each model was adjusted for the following variables: age, gender, co-morbidities (diabetes mellitus, malignancies, leukaemia, lymphoma, connective tissue disease, chronic lung disease, congestive cardiac failure), whether patient had received at least one dose of coronavirus disease 2019 vaccine, and duration on the ward.
More than one-third (34.4%, 22/64) of non-cases had at least one dose of SARS-CoV-2 vaccine, approximately twice the proportion (17.9%, 5/28) in patient cases (P=0.139) (See online supplementary material Table S1). Having had at least one dose of the vaccine was associated with a 79% lower odds of infection (adjusted OR 0.21, 95% CI 0.05–0.95) (Table I ).
Table I.
Odds ratio associated with acquiring coronavirus disease 2019 (COVID-19) among patients on Ward I between 20th and 28th April 2021
| Variable | Univariable logistic regression |
Multiple logistic regression |
||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P-value | Adjusted ORb | 95% CI | P-value | |
| Age (years) | ||||||
| <70 | Reference group | Reference group | ||||
| 70–79 | 1.90 | 0.57–6.32 | 0.292 | 2.00 | 0.48–8.41 | 0.345 |
| 80–89 | 1.25 | 0.38–4.16 | 0.716 | 1.52 | 0.36–6.36 | 0.569 |
| ≥90 | 5.36 | 1.17–24.43 | 0.030 | 6.26 | 0.88–44.5 | 0.067 |
| Gender | ||||||
| Female | Reference group | Reference group | ||||
| Male | 0.75 | 0.31–1.83 | 0.529 | 0.58 | 0.19–1.72 | 0.326 |
| With selected co-morbiditiesa | ||||||
| No | Reference group | Reference group | ||||
| Yes | 2.41 | 0.97–5.99 | 0.058 | 3.09 | 1.00–9.54 | 0.050 |
| Had at least one dose of COVID-19 vaccine | ||||||
| No | Reference group | Reference group | ||||
| Yes | 0.42 | 0.14–1.24 | 0.116 | 0.21 | 0.05–0.95 | 0.043 |
| Days on Ward I between 20th and 28th April 2021 | 1.36 | 1.15–1.61 | <0.0001 | 1.38 | 1.14–1.68 | 0.001 |
OR, odds ratio; CI, confidence interval.
Co-morbidities included were diabetes mellitus, malignancies, leukaemia, lymphoma, connective tissue disease, chronic lung disease and congestive cardiac failure.
Adjusted for all variables in the table.
Ten potential AGPs were performed on five patients on the ward during the risk period, of which six (all nebulizer treatments) were carried out on two patients with COVID-19, while four (two nebulizers, two high-flow oxygen administrations) were performed on three non-COVID-19 cases. All such procedures performed on the two patients with COVID-19 took place at least 48 h prior to symptom onset and were not performed by the two affected nurses.
The nine staff who became infected included two physicians, two Ward I nurses, one physiotherapist, one pharmacist, one housekeeper, one porter and one ED nurse (See online supplementary material Table S2). Similar proportions of staff cases and non-cases had at least one dose of the SARS-CoV-2 vaccine (87.5% vs 87.1%, P=1.000). All ward-based staff cases (N=5, i.e. excluding the two physicians, porter and ED nurse) were close contacts of a case, compared with less than half of the ward-based staff non-cases (P=0.028); and these cases spent significantly longer periods of time on the ward (median 37.0 vs 5.0 h; P=0.024) but duration on the ward was no longer significant after adjustment. The attack rate among ward-based staff was 3.9% (five of 129 staff) (See online supplementary material Table S3).
Six visitors (1.9%) were infected out of 309 visitors to Ward I. The median cumulative duration of visits to Ward I among visitor cases was significantly longer compared with non-cases (335.9 vs 63.0 min; P=0.036), and the median number of visits was also significantly higher (3 vs 1; P=0.025). Compared with non-cases, a higher proportion of visitor cases were close contacts of a patient case [66.7% (4/6) vs 12.2% (37/303); P=0.003]. In adjusted analyses, being a close contact was associated with infection (adjusted OR 12.61, 95% CI 2.15–74.01). Visitors to Cubicle 2 were not at higher odds of acquiring COVID-19 (See online supplementary material Table S4).
Discussion
Containment of this cluster of COVID-19 Delta variant in a general ward of an acute care hospital involved the identification and quarantine of a large number of individuals (2011 in total). The risk of infection was highest for those who were close contacts of cases, and who spent a longer duration on the ward. Bed location was important but, unusually, the highest risk did not occur among those who were in the same cubicle as the primary case. Vaccination reduced the risk of infection.
Contact and short-range droplet/aerosol spread from the index case were the most likely modes of transmission: close contact with a COVID-19 case was associated with increased risk of COVID-19 in visitors, and all Ward-I-based staff cases were close contacts of a patient with COVID-19. However, there were five cases where close contact with a patient with COVID-19 could not be identified: Patient D from Ward II, three staff (including one who spent only 15 min on Ward I), and one visitor to the ward. This suggests the involvement of fomite and/or long-range aerosol transmission in this cluster, the latter of which is corroborated by filter swab studies and is consistent with other reports of aerosol transmission [[17], [18], [19]].
The high similarity of viruses among the cases supports the hypothesis that this cluster is due to a single introduction event. No sequence from other local community cases has been grouped together with this cluster (data not shown), suggesting that outbreak response and control measures were sufficient to prevent wider community transmission.
It is notable that this first COVID-19 nosocomial cluster occurred with the Delta variant, which has been reported to be up to 97% more transmissible than the original strain [20,21]. Having had at least one dose of the vaccine reduced the risk of COVID-19 almost five-fold among Ward I patients, suggesting that mRNA-based vaccines protect against the Delta variant of SARS-CoV-2, and this is consistent with a recent report from Public Health England [22].
Klompas et al. [23] recently described a COVID-19 outbreak in Brigham and Women's Hospital, Boston, USA with similar findings and reported comparable strategies in containing the outbreak. The authors noted several important lessons in their outbreak, including the limitations of admission testing, and the potential value of serial testing to identify infections incubating on admission. The present cluster shared many similarities with theirs, and further emphasized the importance of maintaining a high index of suspicion in patients with suggestive symptoms despite an initial negative test.
The PPE regime used previously at the study hospital had proven effective against the original SARS-CoV-2 strain, but the more-infectious Delta variant appears to require enhanced measures. As a consequence of this outbreak, the hospital stepped up PPE to routine N95 respirators and eye protection for all staff on inpatient wards, introduced entry swabs for all inpatients, and rostered routine SARS-CoV-2 testing for all staff and inpatients. Staff were required to eat alone at designated eating areas, seated at least 2 m apart wherever practicable or with the use of tables with impermeable plastic dividers. With the new understanding and reports about the role of aerosol transmission, and some preliminary airflow studies performed internally that suggested impaired ventilation as a contributory cause, high-speed extraction fans were also installed in all general wards to improve air circulation.
Although many countries in Europe and North America have gradually relaxed community measures as vaccination rates increased, infection rates are starting to rise again, partly due to circulation of the more-infectious Delta variant. With the onset of the winter season, there is a risk of a rapid increase in infection rates in the coming months. The need to reduce nosocomial transmission remains important, even as many countries shift to a ‘live with COVID-19’ or mitigation strategy, as hospital patients are more likely to be vulnerable to severe disease and are also more likely to be unvaccinated due to medical reasons. Investigation of the study cluster highlights several key points: transmission can occur through a mixture of routes, and long-range airborne transmission could play an important role, as reported by other authors [24]; transmission can occur even with very short exposure times; and breakthrough infections and subsequent transmission remain a risk in highly vaccinated environments such as hospital settings, and enhanced measures may be required to prevent nosocomial clusters.
Key limitations of this study are that common and frequent interactions between staff and patients made network analysis unfeasible. In addition, the small number of infected staff of each staff type did not permit delineation of high-risk activities.
In conclusion, this cluster occurred despite the enhancement of infection control measures at the study hospital at the onset of the COVID-19 pandemic. Fortunately, this cluster was brought under control rapidly through case isolation, extensive contact tracing and quarantine measures. Coinciding with the introduction of the Delta variant into Singapore, and occurring after 1.5 years with no nosocomial transmission, this cluster and the subsequent investigations have led to changes in the use of PPE by staff, implementation of routine rostered COVID-19 testing of inpatients and staff, and enhancements to the hospital infrastructure to improve ventilation within general wards.
Acknowledgements
The authors wish to thank Prof. David Allen for reviewing an earlier draft; Dr Ng Lee Ching, Dr Octavia Sophie, Ms Low Swee Ling and Dr Jerald Yam for their assistance with the air-filter swab studies; and colleagues at the Departments of Clinical Epidemiology, Infection Prevention and Control, Laboratory Medicine, Development Support Services, and Hospitality and Environmental Services at Tan Tock Seng Hospital for their contributions to the investigation and management of the cluster.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhin.2021.12.011.
Author contributions
Study conception and design: W-Y Lim, GS-E Tan, BSP Ang and ALP Chow.
Acquisition of data: GS-E Tan, BF Poh, HL Htun, MK Win, H Guo, L Cui, TM Mak, JCC Wong and YX Setoh.
Analysis of data and figures: L Cui, TM Mak, JCC Wong, YX Setoh, HL Htun and HP Phua.
Interpretation of data: W-Y Lim, GS-E Tan, HL Htun, HP Phua, L Cui, BSP Ang and ALP Chow.
Drafting of manuscript: W-Y Lim, GS-E Tan, HL Htun and HP Phua.
Critical revision: W-Y Lim, GS-E Tan, HL Htun, HP Phua, L Cui, BSP Ang and ALP Chow.
All authors read and approved the final manuscript.
Conflict of interest statement
None declared.
Funding sources
None.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.World Health Organization . WHO; Geneva: 2021. Weekly epidemiological update on COVID-19.https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19---11-may-2021 Available at: [last accessed May 2021] [Google Scholar]
- 2.Htun H.L., Lim D.W., Kyaw W.M., Loh W.-N.J., Lee L.T., Ang B., et al. Responding to the COVID-19 outbreak in Singapore: staff protection and staff temperature and sickness surveillance systems. Clin Infect Dis. 2020;71:1947–1952. doi: 10.1093/cid/ciaa468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wong L.Y., Tan A.L., Leo Y.-S., Lee V.J.M., Toh M.P.H.S. Healthcare workers in Singapore infected with COVID-19: 23 January–17 April 2020. Influenza Other Respir Viruses. 2021;15:218–226. doi: 10.1111/irv.12803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chow A., Htun H.L., Kyaw W.M., Lee L.T., Ang B. Asymptomatic health-care worker screening during the COVID-19 pandemic. Lancet. 2020;396:1393–1394. doi: 10.1016/S0140-6736(20)32208-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ho H.J., Lim W.Y., Ang B., Chow A. Use of surveillance technology to enhance exposure management for healthcare workers during the COVID-19 pandemic. J Hosp Infect. 2021;107:101–102. doi: 10.1016/j.jhin.2020.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee C.Y.-P., Amrun S.N., Chee R.S.-L., Goh Y.S., Mak T.-M., Octavia S., et al. Human neutralising antibodies elicited by SARS-CoV-2 non-D614G variants offer cross-protection against the SARS-CoV-2 D614G variant. Clin Transl Immunol. 2021;10:e1241. doi: 10.1002/cti2.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garrison E, Marth G. Haplotype-based variant detection from short-read sequencing. arXiv Preprint 2012;arXiv:12073907.
- 8.Shu Y., McCauley J. GISAID: Global initiative on sharing all influenza data – from vision to reality. Euro Surveill. 2017;22:30494. doi: 10.2807/1560-7917.ES.2017.22.13.30494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Katoh K., Standley D.M. MAFFT Multiple Sequence Alignment Software Version 7: improvements in performance and usability. Mol Biol Evol. 2013;30:772–780. doi: 10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Waterhouse A.M., Procter J.B., Martin D.M.A., Clamp M., Barton G.J. Jalview Version 2 – a multiple sequence alignment editor and analysis workbench. Bioinformatics. 2009;25:1189–1191. doi: 10.1093/bioinformatics/btp033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nguyen L.-T., Schmidt H.A., von Haeseler A., Minh B.Q. IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol Biol Evol. 2014;32:268–274. doi: 10.1093/molbev/msu300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kalyaanamoorthy S., Minh B.Q., Wong T.K.F., von Haeseler A., Jermiin L.S. ModelFinder: fast model selection for accurate phylogenetic estimates. Nat Meth. 2017;14:587–589. doi: 10.1038/nmeth.4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoang D.T., Chernomor O., von Haeseler A., Minh B.Q., Vinh L.S. UFBoot2: improving the ultrafast bootstrap approximation. Mol Biol Evol. 2017;35:518–522. doi: 10.1093/molbev/msx281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention . CDC; Atlanta, GA: 2020. New standard operating procedure for creating viral transport media.https://www.cdc.gov/csels/dls/locs/2020/new_sop_for_creating_vtm.html Available at: [last accessed May 2021] [Google Scholar]
- 15.Centers for Disease Control and Prevention . CDC; Atlanta, GA: 2020. Research use only 2019-novel coronavirus (2019-nCoV) real-time RT-PCR primers and probes.https://www.cdc.gov/coronavirus/2019-ncov/lab/rt-pcr-panel-primer-probes.html Available at: [last accessed May 2021] [Google Scholar]
- 16.Chow A., Guo H., Kyaw W.M., Li A.L., Lim R.H.F., Ang B. Rostered routine testing for severe acute respiratory coronavirus virus 2 (SARS-CoV-2) infection among healthcare personnel – is there a role in a tertiary-care hospital with enhanced infection prevention and control measures and robust sickness-surveillance systems? Infect Control Hosp Epidemiol. 2021 doi: 10.1017/ice.2021.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klompas M., Baker M.A., Rhee C. Airborne transmission of SARS-CoV-2: theoretical considerations and available evidence. JAMA. 2020;324:441–442. doi: 10.1001/jama.2020.12458. [DOI] [PubMed] [Google Scholar]
- 18.Tang J.W., Marr L.C., Li Y., Dancer S.J. COVID-19 has redefined airborne transmission. BMJ. 2021;373:n913. doi: 10.1136/bmj.n913. [DOI] [PubMed] [Google Scholar]
- 19.Greenhalgh T., Jimenez J.L., Prather K.A., Tufekci Z., Fisman D., Schooley R. Ten scientific reasons in support of airborne transmission of SARS-CoV-2. Lancet. 2021;397:1603–1605. doi: 10.1016/S0140-6736(21)00869-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cherian S., Potdar V., Jadhav S., Yadav P., Gupta N., Das M., et al. Convergent evolution of SARS-CoV-2 spike mutations, L452R, E484Q and P681R, in the second wave of COVID-19 in Maharashtra, India. bioRxiv. 2021 doi: 10.3390/microorganisms9071542. 2021.04.22.440932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoffmann M., Hofmann-Winkler H., Krüger N., Kempf A., Nehlmeier I., Graichen L., et al. SARS-CoV-2 variant B.1.617 is resistant to bamlanivimab and evades antibodies induced by infection and vaccination. bioRxiv. 2021 doi: 10.1016/j.celrep.2021.109415. 2021.05.04.442663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bernal J.L., Andrews N., Gower C., Gallagher E., Simmons R., Thelwall S., et al. Effectiveness of COVID-19 vaccines against the B.1.617.2 variant. medRxiv. 2021 2021.05.22.21257658. [Google Scholar]
- 23.Klompas M., Baker M.A., Rhee C., Tucker R., Fiumara K., Griesbach D., et al. A SARS-CoV-2 cluster in an acute care hospital. Ann Intern Med. 2021;174:794–802. doi: 10.7326/M20-7567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tang J.W., Bahnfleth W.P., Bluyssen P.M., Buonanno G., Jimenez J.L., Kurnitski J., et al. Dismantling myths on the airborne transmission of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) J Hosp Infect. 2021;110:89–96. doi: 10.1016/j.jhin.2020.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.