Figure 3. CDK4/6 inhibitor at the crosstalk of cell cycle and immune surveillance.
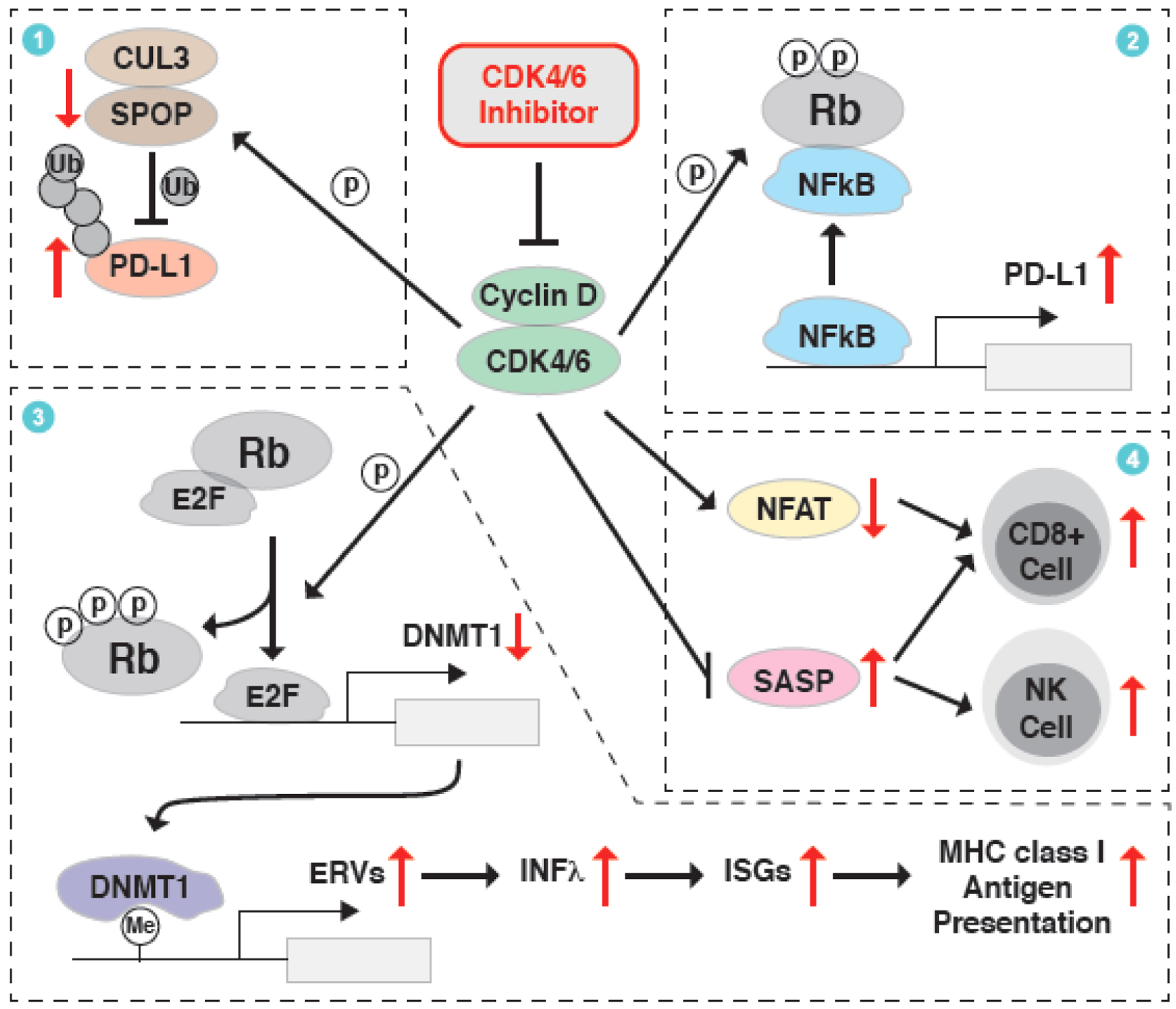
An unexpected immune-regulatory function of CDK4/6 inhibitor has been observed in clinic and various underlying mechanisms have been revealed to contribute to this phenotype. First, Cyclin D-CDK4/6 kinase can phosphorylate and stabilize SPOP, thus facilitating CUL3SPOP E3 ligase-mediated ubiquitination and proteasomal degradation of PD-L1, which could be blocked by the CDK4/6 inhibitor. Second, Cyclin D-CDK4/6 phosphorylates Rb, promotes its binding to NFκB/p65 and thus inactivates the transcription of PD-L1, while CDK4/6 inhibition triggers the transcription of PD-L1. Third, Cyclin D-CDK4/6 phosphorylates Rb to active E2F-mediated transcription of DNMT1, an epigenetic transcription repressor of endogenous retroviral elements (ERVs). This process could be antagonized by CDK4/6 inhibitor to trigger the IFNλ/ISGs/MHC signaling axis and the presentation of tumor antigens. Lastly, inhibition of CDK4/6 stimulates ASAP and represses NFAT-mediated transcriptome, which eventually promotes the infiltration of CD8+ T cells and/or NK cells for the killing of cancer cells.
