Abstract
Recent studies have suggested that 25-hydroxyvitamin D [25(OH)D] may be a poor biomarker of bone health, in part because measured levels incorporate both protein bound and free vitamin D. The ratio of its catabolic product (24,25-dihydroxyvitamin D [24,25(OH)2D]) to 25(OH)D (the vitamin D metabolite ratio or VMR) may provide more information on sufficient vitamin D stores and is not influenced by vitamin D binding protein concentrations. We evaluated whether the VMR or 25(OH)D are more strongly associated with bone loss and fracture risk in older adults. We performed a retrospective cohort study of 786 community dwelling adults aged 70–79 years who participated in the Health Aging and Body Composition study. Our primary outcomes were annual changes in bone density and incident fracture. The mean age of these participants was 75± 3 years, 49% were female, 42% were Black, and 23% had an eGFR<60ml/ml/1.73m2. In fully adjusted models, a 50% lower VMR was associated with 0.3% (0.2%, 0.6%) more rapid decline in total hip BMD. We found similar relationships with thoracic and lumbar spine BMD. In contrast, 25(OH)D3 concentrations were not associated with longitudinal change in BMD. There were 178 fractures during a mean follow-up of 10 years. Each 50% lower VMR was associated with a 49% (95% CI 1.06, 2.08) greater fracture whereas lower 25(OH)D3 concentrations were not significantly associated with fracture risk [HR per 50% lower 1.07 (0.80, 1.43)]. In conclusion, among a diverse cohort of community dwelling older adults, a lower VMR was more strongly associated with both loss of BMD and fracture risk compared to 25(OH)D3. Trials are needed to evaluate the VMR as a therapeutic target in persons at risk for worsening BMD and fracture.
Keywords: DXA, PTH/Vit D/FGF23, Osteoporosis, Fracture Prevention
INTRODUCTION
Multiple studies have suggested that vitamin D deficiency is highly prevalent in the U.S. and severe vitamin D deficiency is known to lead to osteomalacia and fractures.1,2 However, recent observational studies and randomized trials suggest that serum 25-hydroxyvitamin D [25(OH)D] may be a poor biomarker of vitamin D status and bone health.3,4,5 New biomarkers of vitamin D status and their relationship with health and bone outcomes are currently being evaluated.
The active form of vitamin D is 1,25-dihydroxyvitamin D [calcitriol or 1,25(OH)2D]. 1,25(OH)2D is a potential vitamin D biomarker as it is the hormone that binds the vitamin D receptor (VDR), unlike 25(OH)D. However, 1,25(OH)2D has a short half-life (around 5–8 hours) and thus concentrations vary considerably over the day.6,7 Additionally, 1,25(OH)2D circulates at concentrations much lower than 25(OH)D making accurate measurement difficult.6 For these reasons, 1,25(OH)2D may not be a reliable biomarker of vitamin D status. Both 25(OH)D and 1,25(OH)2D are bound by vitamin D binding protein (VDBP) in circulation, thus an additional challenge evaluating both of these biomarkers are that the assays measure both free and protein bound vitamin D metabolites.
Vitamin D catabolism, from 25(OH)D to 24,25-dihydroxyvitamin D [24,25(OH)2D], via the CYP24A1 enzyme, is stimulated by 1,25(OH)2D activating the VDR, preventing the occurrence of vitamin D toxicity.8,9 In fact, persons with CYP24A1 deficiency can develop profound hypercalcemia, suggesting that this pathway is needed to prevent excess calcitriol concentrations.10 Therefore, in persons with a functional CYP24A1 enzyme, as VDR activity increases, so do concentrations of 24,25(OH)2D. Considering this feedback loop, 24,25(OH)2D has been suggested as a surrogate biomarker of VDR activity, even though 24,25(OH)2D, like 25(OH)D, is not thought to be biologically active. 24,25(OH)2D measurement does not suffer from the difficulties of 1,25(OH)2D measurement as it circulates in much higher concentrations and has a longer half-life, both of which are comparable to that of 25(OH)D. Unfortunately, interpretation of all vitamin D metabolite measurements remains difficult without knowledge of vitamin D binding protein concentrations (VDBP).11 Total vitamin D concentrations largely reflectVDBP concentrations, as 25(OH)D, 24,25(OH)2D, and 1,25(OH)2D are all mostly bound to VDBP; yet VDBP does not appear to affect bioavailable vitamin D levels.12 The ratio of 24,25(OH)2D to 25(OH)D (vitamin D metabolite ratio or VMR) is a biomarker of vitamin D status that is theoretically increased by calcitriol binding to the VDR leading to increased VDR activity. We recently demonstrated the VMR is independent of variability in VDBP.12 These findings have led to increased interest in using the VMR as a superior biomarker of vitamin D status.
Preliminary studies have compared the relationships of the VMR vs. 25(OH)D with bone and health outcomes. Some studies have suggested that the VMR may have stronger associations with risk of hip fracture and death relative to that of 25(OH)D.3,13 To our knowledge, the relationship of the VMR with longitudinal changes in bone mineral density (BMD) and overall fractures has not yet been tested.
To that end, in the present study, we evaluate the relationship between vitamin D metabolites (including 25(OH)D, 24,25(OH)2D, 1,25(OH)2D and the VMR) with changes in BMD at several anatomic regions, as well as risk of fractures in a cohort of community dwelling older individuals in the Health Aging and Body Composition (ABC) study. Additionally, as it is uncertain whether any differences in relationships with outcomes may be due to different VDBP concentrations, we measured VDBP as well. Our a priori hypothesis was that a lower VMR would be more strongly associated with a lower BMD and higher fracture risk than 25(OH)D. Additionally, we hypothesized that the relationship of vitamin D metabolites with changes in BMD and fracture risk would be strengthened when accounting for the confounding influences of VDBP concentrations and isoform. We hypothesize that these relationships reflect underlying VDR activity, and not that the VMR or its metabolites therein are causative of these beneficial effects on bone density and fracture risk.
METHODS
Study Population
The Health ABC study was a longitudinal cohort designed to evaluate relationships between body composition health outcomes in community-dwelling older adults.5 Between April 1997 and June 1998, 3,075 well-functioning older adults between the age of 70 and 79 years were recruited from Memphis, TN and Pittsburgh, PA. Participants were recruited from a random sample of Medicare beneficiaries.14 Participants were required to be able to walk at least one quarter mile without difficulty, climb 10 steps, and perform basic activities of daily living. The study was approved by the local institutional review boards (IRBs) and informed consent was obtained from all Health ABC participants. The present study was also approved by the IRB at the University of California San Diego.
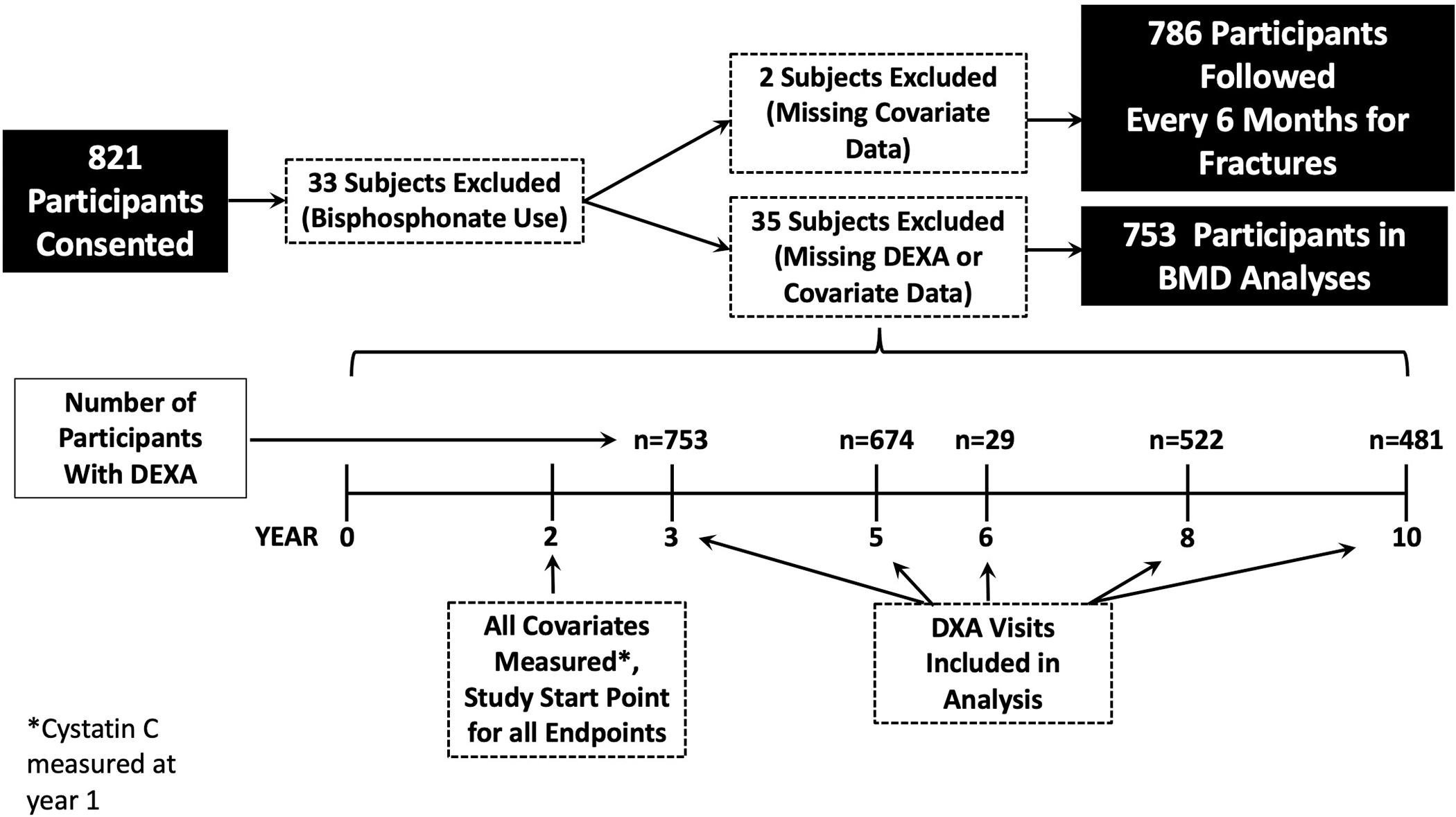
Vitamin D metabolites including 25(OH)D2, 25(OH)D3, 24,25(OH)2D3, 1,25(OH)2D2, and 1,25(OH)2D3 were measured in 821 individuals using samples collected at the year two Health ABC visit. We excluded 33 subjects that received bisphosphonate therapy for osteoporosis as this was thought to likely bias the results. Additionally two other subjects were excluded due to missing vitamin D data. These participants comprised a random subcohort of 478 participants, and 308 additional participants chosen because they had subsequently developed declining kidney function, defined as an eGFR decline of ≥30% from baseline based on serial cystatin C measurements and the CKD-EPI cystatin C formula.13 Accurate Vitamin D binding protein concentrations were obtained in a subset of 377 of these 786 individuals.12
Exposure Variables
Participants had fasted for ≥ 8 hours at the time of blood sampling. Samples were stored at −70°C from collection in 1998–1999 (year 2 visit) until testing. Considering the lack of spectroscopic evidence of 24,25(OH)2D2, our analyses focused on the D3 metabolites. The exposure variables in our primary analysis were 25(OH)D3, 24,25(OH)2D3, and 1,25(OH)2D3, along with the VMR, calculated by dividing serum 24,25(OH)2D3 by serum 25(OH)D3 and then multiplying by 100.3 The intraassay coefficient of variation for 25(OH)D3, 24,25(OH)2D3, and 1,25(OH)2D3 assays are <5.6%, 9.9–12.7% and 5.6–8%, respectively. Each metabolites concentration was quantified using immunoaffinity enrichment and liquid chromatography-tandem mass spectrometry, as described previously.13
Outcome Variables
Change in total hip BMD served as our primary outcome. BMD (g/cm2) of the hip (proximal femur), thoracic spine and lumbar spine were assessed by dual X-ray absorptiometry (DXA) (Hologic 4500A, version 8.20a, Waltham, MA). BMD measurements were performed at the baseline and year 3, 5, 6, 8 and 10 visits.15 DXA quality assurance measurements were performed at both study sites to ensure that scanner reliability and identical patient scan protocols were employed for all participants. We evaluated changes in BMD as the mean annual percent change in BMD for each BMD site, starting from the first visit after vitamin D measurements.
We additionally evaluated time to first fracture. Incident fractures were assessed every 6 months by self-report and validated by radiology reports. Fractures were categorized by site and time to event (starting from time of vitamin D measurements), and fractures at any site were included in this analysis. The mean follow-up time for the fracture outcome was 10 years.
Other Measurements
All participants provided a medical history and physical examination. We recorded the clinical site and the season of blood measurement as these parameters can influence vitamin D measurements. Age, sex, race, and smoking status were determined by self-report. Body mass index (BMI) was calculated in kg/m2. Baseline prevalent diabetes was either self-reported or determine by the use of antidiabetic agents, fasting glucose concentration ≥ 126 mg/dL, or a 2-hour oral glucose tolerance test result ≥ 200 mg/dL. Study staff categorized medication data using the Iowa Drug Information System.
VDBP concentration and isoform were determined simultaneously via liquid chromatography-tandem mass spectrometry, as described previously with minor assay modification.16 Intraassay coefficient of variation for the VDBP assay ranges from 3.1 to 9.1% across a range of concentrations. After measurement of VDBP we noted measurement problems with certain samples and therefore excluded any measurements that did not meet our internal quality metrics.12 Therefore, for our regression models using VDBP (model 3), our cohort was limited to 377 participants. All other models did not include VDBP (as discussed below), and therefore, 821 participants were included.
Serum cystatin C measurements were available only at year 1 (as opposed to year 2 concurrent with measurement of vitamin D metabolites and all other measurements included in this analysis). Thus, we applied the year-1 cystatin C measurements to year 2.5 Cystatin C was measured at the core laboratory (University of Vermont, Burlington, VT) using a particle-enhanced immunonephelometric assay (N Latex Cystatin C). Serum calcium and phosphate were measured using direct quantitative colorimetric determination (Stanbio Laboratory). We estimated GFR (eGFR) using the 2012 CKD Epidemiology Collaboration (CKD-EPI) cystatin C equation.17 Intact PTH (iPTH) was measured in EDTA plasma using a 2-site immunoradiometric assay kit (N-tact PTHSP; DiaSorin). FGF-23 was measured using an intact assay (Kainos Laboratories). Serum albumin was measured using the same assay for VDBP.13
Statistical Methods
In order to account for the sampling of the kidney function decline cases, incident CKD cases arising outside the random subcohort were given a weight of 1, and subcohort participants were weighted by the inverse probability of their sampling fraction to re-create a random subcohort and to avoid biasing the sample. We then used linear mixed models with random intercepts and slopes to estimate and compare linear trends of to assess the associations of 24,25(OH)2D3, 25(OH)D3, 1,25(OH)2D3, the VMR as well as PTH with annual change in total hip BMD. To facilitate comparisons, we log transformed both the exposure and outcome variables such that beta coefficients are interpretable as the annual percentage change in hip BMD attributable to a 50 percent lower vitamin D metabolite or VMR at baseline. We developed a sequence of models. Model 1 was adjusted for age, sex, race, season of blood sampling, clinic site and BMI. Model 2, our primary analytic model, was additionally adjusted for eGFR, serum calcium, phosphate, PTH and FGF-23 concentrations. Finally, we created a 3rd model to assess if the associations were altered by inclusion of VDBP concentration, isoform, and serum albumin concentration. This model was only of the smaller, “VDBP trusted” subcohort.12 We also assessed for sex, race and eGFR interactions by inclusion of multiplicative interaction terms in Model 2. In companion analyses, we explored the associations of the same vitamin D metabolites and the VMR with annual changes in thoracic and lumbar spine BMD using the same models as the total hip BMD analysis mentioned above. We also explored the cross-sectional relationship of the vitamin D metabolites and the VMR with baseline PTH concentrations using the same models as stated above, with the exception that the models did not adjust for PTH, as this was the outcome variable.
Lastly, we used Cox models to assess the association between vitamin D metabolites, the VMR, and PTH with time to first fracture using the same sequence of staged models as the BMD analyses. We developed spline functions using additive models, adjusted for Model 2 covariates. The extreme 2.5% of vitamin D measurements were excluded from the spline graphs to avoid implausible extrapolations from the extremes of the data distribution. We conducted all of our analyses in Stata SE version 14.1 (College Station, TX). P-values < 0.05 were considered statistically significant for all analyses including multiplicative-interaction terms.
RESULTS
The mean age of the 786 individuals was 75 years, 52% were women, 42% were African American, 58% were non-Hispanic Caucasian, and 23% had eGFR < 60 ml/min/1.73 m2 at baseline. The mean VMR, 25(OH)D3, 24,25(OH)2D3, and 1,25(OH)2D3 concentrations were 9.3±4 (ng/ml)/(ng/ml), 21±10 ng/ml, 2.1±1.5 ng/ml, and 41±16 pg/ml, respectively. The mean VDBP was 257 ± 41 ug/ml. Baseline characteristics across quartiles of VMR are shown in Table 1. Compared to persons in the lowest VMR quartile, those with higher VMR were more often male and Caucasian, and were less likely to have hypertension, diabetes, CKD, and albuminuria. The VMR was highest in the summer and fall and lowest in winter and spring.
Table 1.
Baseline Characteristics by Vitamin D Metabolite Ratio Quartiles among 786 Older Adult Participants in the Health ABC Study
| Quartile 1 (n= 219) | Quartile 2 (n=194) | Quartile 3 (n= 182) | Quartile 4 (n=191) | |
|---|---|---|---|---|
| Range (ng/ml/ng/ml) | 0.91–6.68 | 6.69–9.47 | 9.50–11.90 | 11.91–30.50 |
|
| ||||
| Age (years) ± SD | 75 (3) | 74(3) | 75(3) | 75 (3) |
| Male, n (%) | 88 (40) | 105 (54) | 104 (57) | 108 (57) |
| Black, n (%) | 136 (62) | 81 (42) | 54 (30) | 59 (31) |
| Clinic site, n(%) | ||||
| Memphis | 120 (55) | 97 (50) | 89 (49) | 98 (51) |
| Pittsburgh | 99 (45) | 97 (50) | 93 (51) | 93 (49) |
| Season of blood measurement, n (%) | ||||
| Winter | 61 (28) | 45 (23) | 46 (25) | 43 (23) |
| Spring | 79 (36) | 51 (26) | 34 (19) | 37 (19) |
| Summer | 35 (16) | 53 (28) | 51 (28) | 50 (26) |
| Fall | 44 (20) | 45 (23) | 51 (28) | 61 (32) |
| BMI (kg/m2) ± SD | 28 (5) | 28(5) | 27 (4) | 26 (4) |
| Smoking status, n (%) | ||||
| Never | 100 (46) | 82 (42) | 80 (44) | 74 (40) |
| Former | 94 (43) | 95 (49) | 89 (49) | 103 (54) |
| Current | 25 (11) | 17 (9) | 13 (7) | 14 (7) |
| Diabetes, n (%) | 102 (46) | 95(49) | 67 (37) | 66 (35) |
| Use of anti-hypertensive medications, n (%) | 148 (67) | 130 (67) | 101 (56) | 113 (59) |
| CKD, n (%) | 78 (36) | 45 (23) | 37 (20) | 24 (13) |
| eGFR (ml/min/1.73m2) ± SD | 66 (23) | 73 (17) | 74 (17) | 80 (16) |
| Albumin/Creatinine, median [IQR] | 10 [5–61] | 8 [4–23] | 7 [4–16] | 8 [4–19] |
| Calcium (mg/dl) ± SD | 8.9 (0.5) | 8.9 (0.4) | 8.8 (0.4) | 8.9 (0.4) |
| Phosphate (mg/dl) ± SD | 3.6 (0.5) | 3.5 (0.5) | 3.5 (0.5) | 3.6 (0.5) |
| Parathyroid hormone (pg/ml), median [IQR] | 45 [34–71] | 33 [25–43] | 29 [23–39] | 27 [21–36] |
| FGF23 (RU/ml), median [IQR] | 44 [32–60] | 45 [32–59] | 45 [36–59] | 46 [36–61] |
| Incident CKD Cases n (%) | 91 (44) | 74 (39) | 66 (36) | 76 (40) |
Body mass index (BMI), Chronic Kidney Disease (CKD), Fibroblast growth factor 23 (FGF23)
There were 753 participants who had BMD measurements for analysis. The median [IQR] total hip BMD was 0.89 [0.79, 1.01] g/cm2. In our demographics adjusted model, a 50% lower VMR was associated with a 0.3% annual faster decrease in total hip BMD (95% CI, 0.2%, 0.6%). This association was essentially unchanged in our fully adjusted model (Table 2). We did not find a statistically significant association of 25(OH)D3 or 1,25(OH)2D3 with annual decline in total hip BMD [0.2% (−0.01%, 0.4%) and 0.1% (−0.1%, 0.3%) per 50% lower levels, respectively]. A 50% lower 24,25(OH)2D3 was associated with an 0.2%/year (95% CI, 0.1%, 0.3%) faster decrease in total hip BMD. Results were similar for changes in thoracic and lumbar BMD (Supplemental Table 1). The addition of VDBP to our models did not materially improve the strength of associations between vitamin D metabolites and any of the BMD outcomes (Supplemental Table 2). Higher baseline PTH concentrations were associated with a decline in total hip, but not thoracic or lumbar BMD (Supplemental Table 3). There were no significant interactions between any of the metabolites with longitudinal changes in total hip BMD by sex, race. or eGFR (p interactions >0.07 for all).
Table 2:
Association of Vitamin D Metabolites and the VMR with Percent Annual Change in Total Hip BMD among 753 participants of the Health ABC Cohort
| VMR | 25D3 | 24,25D3 | 1,25D3 | |||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Annual Change in BMD Per 50% Lower (95%CI) | P | Annual Change in BMD Per 50% Lower (95%CI) | P | Annual Change in BMD Per 50% Lower (95%CI) | P | Annual Change in BMD Per 50% Lower (95%CI) | P | |
|
| ||||||||
| Model 1 | −0.3% (−0.6%, −0.2%) | <0.001 | −0.2% (−0.4%, 0.01%) | 0.066 | −0.2% (−0.3%, −0.1%) | 0.001 | −0.1% (−0.3%, 0.1%) | 0.537 |
| Model 2 | −0.3% (−0.6%, −0.2%) | <0.001 | −0.2% (−0.4%, 0.01%) | 0.066 | −0.2% (−0.3%, −0.1%) | 0.001 | −0.1% (−0.3%, 0.1%) | 0.536 |
Model 1 Adjusted for age, sex, race, season of measurement, clinic site and BMI
Model 2 Additionally adjusted for baseline eGFR, serum calcium, phosphate, parathyroid hormone and fibroblast growth factor 23
Among the 786 participants with baseline vitamin D measurements, there were 178 fractures over a mean follow up time of 10 ± 5 years. The sites of these fracture are reported in Supplemental Table 4 with the most common fracture sites occuring in the arms and hip. In our demographics adjusted model, a 50% lower VMR was associated with a 37% (95% CI 6%, 77%) higher risk of fracture (Table 3). This association was strengthened in our fully adjusted model such that a 50% lower VMR was associated with a 49% (95% CI 6%, 108%) higher risk of fracture. Associations of all other vitamin D metabolites with fracture risk did not reach statistical significance across the sequence of models. Spline functions excluding the extreme 2.5% of measurements are shown in Figure 2, demonstrating a linear increase in fracture risk with a lower VMR. Baseline PTH concentrations were also not associated with fracture risk [HR per doubling of PTH 1.09 95% CI (0.86, 1.38)]. The addition of VDBP to models did not materially change these associations. (Supplemental Table 5). relationships between vitamin D metabolites and the VMR with fracture were similar by sex, race and eGFR (P-interactions >0.06 for all).
Table 3.
Association of Vitamin D Metabolites and the VMR with Fracture Risk among 788 participants of the Health ABC Cohort
| VMR | 25D3 | 24,25D3 | 1,25D3 | |||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| HR Per 50% Lower (95%CI) | P | HR Per 50% Lower (95%CI) | P | HR Per 50% Lower (95%CI) | P | HR Per 50% Lower (95%CI) | P | |
|
| ||||||||
| Model 1a | 1.37 (1.06, 1.78) | 0.017 | 1.09 (0.83, 1.44) | 0.525 | 1.15 (0.98, 1.35) | 0.087 | 1.08 (0.84, 1.40) | 0.545 |
| Model 2b | 1.49 (1.06, 2.08) | 0.021 | 1.07 (0.80, 1.44) | 0.649 | 1.16 (0.96, 1.40) | 0.131 | 1.20 (0.89, 1.62) | 0.237 |
Model 1 Adjusted for age, sex, race, season of measurement, clinic site and BMI
Model 2 Additionally adjusted for baseline eGFR, serum calcium, phosphate, parathyroid hormone and fibroblast growth factor 23
Figure 2: Spline Functions Depicting the Relationship of Vitamin D Metabolites and the VMR with Risk of Fracture.
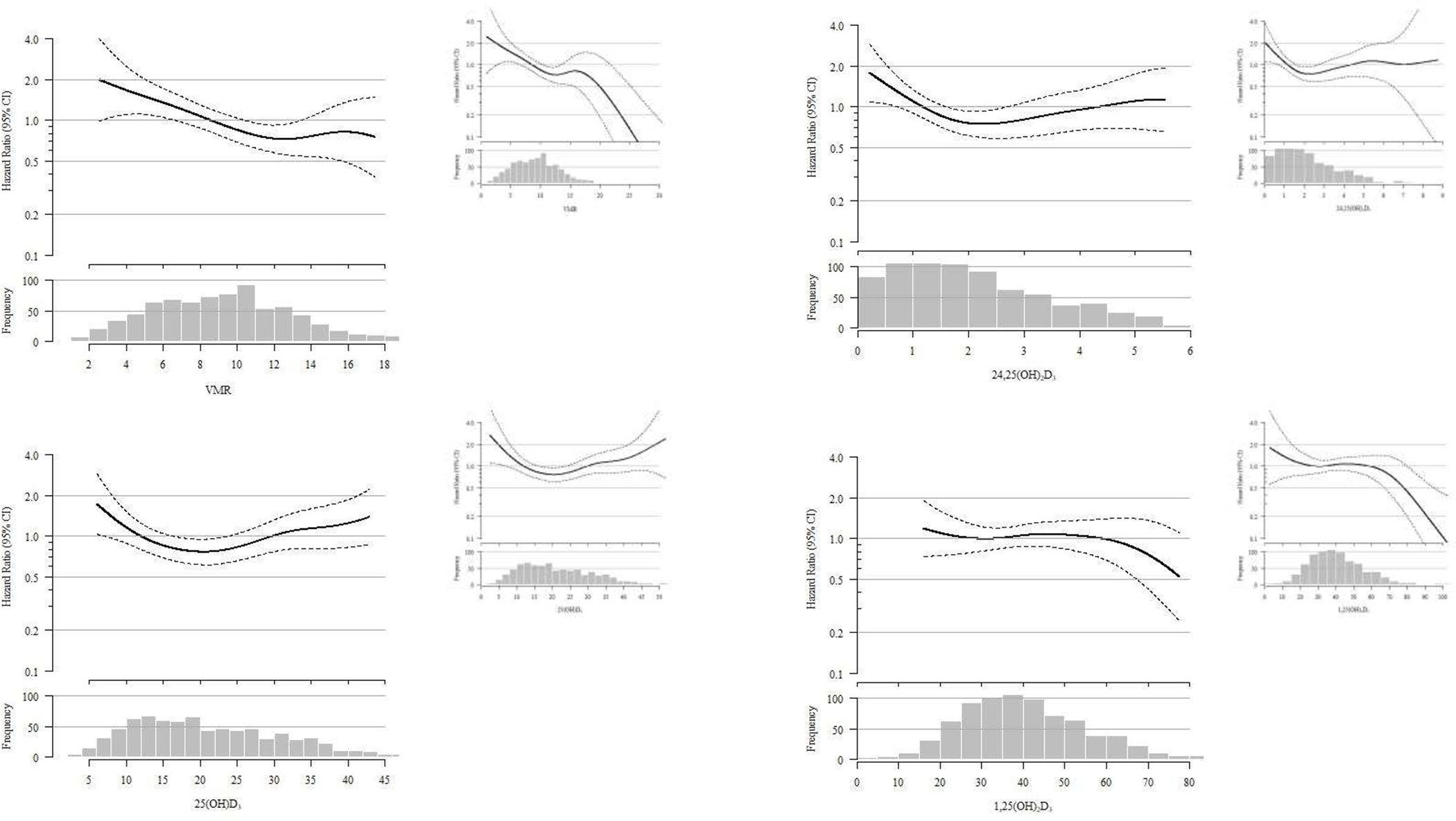
Spline functions exclude the extreme 2.5% on both ends. Solid line represents hazard ratio and dashed lines represent 95% confidence intervals. Spline functions that include the extreme 2.5% values included in figure inset.
Among the 786 participants with concurrent vitamin D metabolites and PTH measurements we found that 25(OH)D, 24,25(OH)2D3, and the VMR were all inversely associated with serum PTH concentrations (Supplemental Table 6). However, serum 1,25(OH)2D3 concentrations were positively associated with PTH concentrations.
DISCUSSION
This study compared the relationship between 25(OH)D and the VMR with longitudinal changes in BMD and fracture risk in a multi-racial cohort of community-living older men and women. We found that a lower VMR had stronger associations with longitudinal declines in BMD and fracture risk compared to 25(OH)D. Results appeared similar in men and women, blacks and whites, and across the range of eGFR.
We and others have evaluated the association of vitamin D metabolites and the VMR with BMD using a single BMD measurement at a single anatomic site.3,18 In our prior cross-sectional analysis of older adults in the Cardiovascular Health Study (CHS), we found no association between the VMR or 25(OH)D with BMD in community living older adults in cross-section, although there was a weak but statistically significant association between 24,25(OH)2D3 with total hip BMD. Similarly, Van Ballegoojien at al. found a weak association between categories of 24,25(OH)2D3 and BMD among 1,773 participants in the Multi-Ethnic Study of Atherosclerosis that approached but did not reach statistical significance (p=0.058).18 Overall, our findings confirm these prior findings that 24,25 may strengthen BMD associations relative to 25D, but importantly, we demonstrate that the VMR appears more strongly associated with BMD than any individual vitamin D metabolite. We extend these findings in several important ways. First, this study evaluates longitudinal trajectories of BMD over time, which may be more informative about ongoing bone changes and fracture risk, and offer stronger inferential evidence than prior cross-sectional studies. We also link the relationships of VMR with BMD and with fracture risk in the same study sample, and demonstrate that lower values of the VMR consistently detected longitudinal changes in BMD at multiple anatomic sites, and with fracture risk, that were missed by the individual metabolites. In comparison, associations of 25(OH)D, which currently serves as the standard clinical biomarker, were not statistically significantly associated with BMD at any site, consistent with multiple prior reports.3,11,19,20 Nonetheless, considering this is the first study to evaluate the VMR and longitudinal BMD changes, these findings need to be validated in other cohorts with serial BMD measurements.
An important sub-hypothesis for this study was that the relationship of the individual vitamin D metabolites with clinical outcomes would be strengthened when adjusting for VDBP concentrations in our models. Considering that the VMR is not dependent on VDBP concentrations,12 we hypothesized VDBP would not materially alter the relationship between the VMR and the outcomes studied. Unfortunately, we only obtained reliable VDBP measurements in a subset of participants which limited statistical power for testing this hypothesis. Nonetheless, contrary to our hypothesis, we found that inclusion of VDBP did not meaningfully influence associations of vitamin D metabolites with outcomes. We speculate that this may indicate that the limitations of 25(OH)D and 1,25(OH)2D3 as biomarkers of bone health may be larger than simply being confounded by differences in VDBP.
The reasons for the associations of the VMR with bone health outcomes is yet to be completely elucidated. We theorized that one reason calcitriol may serve as a poor biomarker of bone health may be due to low concentrations in blood, limiting accurate measurement, as well as a short half-life. The effects of calcitriol on mineral metabolism are well demonstrated, yet concentrations do not consistently predict clinical outcomes, as in our study.21,22 One approach to this problem in medicine has been to monitor the upstream metabolites involved in a negative feedback loop to gain further insight into the active hormone of interest. For example, monitoring of thyroid function depends on measurement of thyroid stimulating hormone, as it inhibited by free thyroxine (the active hormone). Similarly, we suggest measuring the upstream metabolites of the vitamin D negative feedback loop, specifically measuring 24,25(OH)2D and calculating the VMR, to provide information on the active hormone (calcitriol binding to the VDR). However, an alternative hypothesis is that 24,25(OH)2D is in fact biologically active, and this may be the reason why the VMR is strongly associated with changes in BMD and fracture. In vitro and animal studies have suggested this in fact may be the case.23,24 Further studies are needed to determine if 24,25(OH)2D is itself biologically active, or simply a biomarker of VDR activity.
This study has several strengths. First, we evaluated a cohort of older individuals, including both men and women and black and white elders, who collectively represent a population at high risk for bone disease and fracture. Second, we evaluated associations of baseline vitamin D measures with serial bone density measurements over years, at multiple anatomic sites, and with fracture outcomes concurrently. We evaluated whether results differed by severity of kidney function. Lastly, this study has an extensive set of vitamin D measurements, made concurrently, including 25(OH)D, 24,25(OH)2D, 1,25(OH)2D3 and VDBP.
This study also has important limitations. First, our VDBP analyses were limited to a subset due to measurement concerns in the remainder, as detailed elsewhere.12 Second, the number of fractures that occurred during follow-up were too few to evaluate specific fracture sites separately. However, the consistency of our findings with both longitudinal changes in BMD and fracture risk suggests our findings are robust. Third, while this cohort had a significant number of participants with CKD, very few had advanced CKD. Considering vitamin D catabolism is reduced in CKD, this may limit the generalizability of these findings in persons with advanced CKD.25 Similarly, the cohort was a fairly healthy sample with a narrow age range, based on the inclusion criteria, and we excluded women who were receiving bisphosphonates. Fourth, our longitudinal BMD analysis may have been impacted by survivor bias, as the relationship of the vitamin D metabolites with changes in BMD may have been different among participants that died prior to serial BMD measurements. Last, this was not an interventional study. Thus, it remains unclear if VMR changes translate to changes in fracture risk; an important question before the VMR could be utilized as an intermediate treatment target. Nonetheless, multiple studies have demonstrated that the VMR can be increased or decreased in response to therapies like vitamin D supplementation or intestinal phosphate binders.26,27
In summary, we demonstrate that a lower VMR is associated with longitudinal declines in BMD at the hip, thoracic and lumbar spine and increased risk of fractures in community-living older adults. In contrast, 25(OH)D, the current clinical marker of vitamin D status, was not associated with any of the bone health outcomes. In light of these observations, the VMR may be a more reliable indicator of vitamin D sufficiency and bone health than 25(OH)D concentrations in older adults. Trials are needed to determine if the VMR can be used as an intermediate therapeutic target in addition to a biomarker of bone health.
Supplementary Material
Kidney Function declined as a decline in eGFR by of ≥30% from baseline
Figure 1: Study Sample for Fracture and Change in BMD Outcomes.
ACKNOWLEDGEMENTS
The authors acknowledge the services of the Health ABC Study, contributing research centers, and all study participants.
Support: This study was supported by grants from the National Institute of Diabetes, Digestive, and Kidney Diseases K23DK118197 and Loan Repayment Program (Dr. Ginsberg), R01DK101720 and K24 DK110427 (Dr. Ix), the National Institute on Aging (NIA) 5R01AG027002 (Drs. Sarnak and Shlipak), R01AG033087 (Dr. Kritchevsky, and University of Washington Nutrition and Obesity Research Center P30DK035816 (Dr. Hoofnagle). Dr. Ix was additionally supported by an Established Investigator Award from the American Heart Association (14EIA18560026) This research was additionally supported by National Institute on Aging (NIA) Contracts N01-AG-6-2101; N01-AG-6-2103; N01-AG-6-2106; NIA grant R01-AG028050, and NINR grant R01-NR012459. This research was funded in part by the Intramural Research Program of the NIH, National Institute on Aging. Funders did not have a role in study design, data collection, analysis, reporting, or the decision to submit for publication.
Pimary Funding Source: The National Institute of Diabetes, Digestive, and Kidney Diseases, the National Heart, Lung and Blood Institute, the National Institute on Aging, and the American Heart Association.
Data Availability Statement
The data that support the findings of this study are available from Health ABC. Restrictions apply to the availability of these data, which were used under license for this study. Data are available https://healthabc.nia.nih.gov with the permission of Health ABC.
REFERENCE
- 1.Bischoff-Ferrari HA, Dietrich T, Orav EJ, Dawson-Hughes B. Positive association between 25-hydroxy vitamin D levels and bone mineral density: a population-based study of younger and older adults. Am J Med 2004. May 1;116(9):634–9 [DOI] [PubMed] [Google Scholar]
- 2.Priemel M, von Domarus C, Klatte TO, Kessler S, Schlie J, Meier S, Proksch N, Pastor F, Netter C, Streichert T, Püschel K, Amling M. Bone mineralization defects and vitamin D deficiency: histomorphometric analysis of iliac crest bone biopsies and circulating 25-hydroxyvitamin D in 675 patients. J Bone Miner Res 2010. February;25(2):305–12 [DOI] [PubMed] [Google Scholar]
- 3.Ginsberg C, Katz R, de Boer IH, Kestenbaum BR, Chonchol M, Shlipak MG, Sarnak MJ, Hoognagle AN, Rifkin DE, Garimella PS, Ix JH, The 24,25 to 25-Hydroxyvitamin D Ratio and Fracture Risk in Older Adults: The Cardiovascular Health Study Bone. 2018. February;107:124–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burt LA, Billington EO, Rose MS, Raymond DA, Hanley DA, Boyd SK. Effect of High-Dose Vitamin D Supplementation on Volumetric Bone Density and Bone Strength: A Randomized Clinical Trial. J AMA. 2019. August 27;322(8):736–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.LeBoff MS, Chou SH, Murata EM, Donlon CM, Cook NR, Mora S, Lee IM, Kotler G, Bubes V, Buring JE, Manson JE. Effects of Supplemental Vitamin D on Bone Health Outcomes in Women and Men in the VITamin D and OmegA-3 TriaL (VITAL). J Bone Miner Res 2020. May;35(5):883–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Souberbielle JC, Cavalier E, Delanaye P, Massart C, Brailly-Tabard S, Cormier C, Borderie D, Benachi A, Chanson P. Serum calcitriol concentrations measured with a new direct automated assay in a large population of adult healthy subjects and in various clinical situations. Clin Chim Acta 2015. December 7;451(Pt B):149–53. [DOI] [PubMed] [Google Scholar]
- 7.Holick MF, Binkley NC, Bischoff-Ferrari HA, Massart C, Brailly-Tabard S, Cormier C, Borderie D, Benachi A, Chanson P Endocrine Society. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2011. July;96(7):1911–30 [DOI] [PubMed] [Google Scholar]
- 8.Jones G. Pharmacokinetics of vitamin D toxicity. Am J Clin Nutr August; 2008. 88(2):582S–586S [DOI] [PubMed] [Google Scholar]
- 9.Tryfonidou MA, Oosterlaken-Dijksterhuis MA, Mol JA, van den Ingh TS, van den Brom WE, Hazewinkel HA. 24-Hydroxylase: potential key regulator in hypervitaminosis D3 in growing dogs. Am J Physiol Endocrinol Metab 2003. March; 284(3):E505–13 [DOI] [PubMed] [Google Scholar]
- 10.Cavalier E, Huyghebaert L, Rousselle O, Bekaert AC, Kovacs S, Vranken L, Peeters S, Le Goff C, Ladang A. Simultaneous measurement of 25(OH)-vitamin D and 24,25(OH)2-vitamin D to define cut-offs for CYP24A1 mutation and vitamin D deficiency in a population of 1200 young subjects. Clin Chem Lab Med. 2020. January 28;58(2):197–201. doi: 10.1515/cclm-2019-0996. PMID: 31804956. [DOI] [PubMed] [Google Scholar]
- 11.Powe CE, Ricciardi C, Berg AH, Erdenesanaa D, Collerone G, Ankers E, Wenger J, Karumanchi SA, Thadhani R, Bhan I. Vitamin D-binding protein modifies the vitamin D-bone mineral density relationship J. Bone Miner. Res, 26 (July (7)) (2011), pp. 1609–1616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ginsberg C, Hoofnagle AN, Katz R, Becker JO, Kritchevsky SB, Shlipak MG, Sarnak MJ, Ix JH. The Vitamin D Metabolite Ratio is Independent of Vitamin D Binding Protein Concentration. Clin. Chem 2020, In Press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Selamet U, Katz R, Ginsberg C, Rifkin DE, Fried LF, Kritchevsky SB, Hoofnagle AN, Bibbins-Domingo K, Drew D, Harris T Newman A, Gutiérrez OM, Sarnak MJ, Shlipak, Ix JH, Serum Calcitriol Concentrations and Kidney Function Decline, Heart Failure, and Mortality in Elderly Community-Living AdultsPersons: The Health, Aging and Body Composition Study. Am J Kidney Dis 2018. September;72(3):419–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fredman L, Cauley JA, Satterfield S, Simonsick E, Spencer SM, Ayonayon HN, Harris TB; Health ABC Study Group. Caregiving, mortality, and mobility decline: the Health, Aging, and Body Composition (Health ABC) Study. Arch Intern Med 2008. October 27;168(19):2154–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lloyd J, Alley D, Hochberg M et al. Changes in bone mineral density over time by body mass index in the Health ABC Study. Osteoporos Int. 2016;27:2109–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Henderson CM, Lutsey PL, Misialek JR, et al. Measurement by a Novel LC-MS/MS Methodology Reveals Similar Serum Concentrations of Vitamin D-Binding Protein in Blacks and Whites Clin Chem 2016. January;62(1):179–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Inker LA, Schmid CH,Tighiouart H, et al. CKD-EPI Investigators. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med 2012;367(1):20–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Ballegooijen AJ, Robinson-Cohen C, Katz R, et al. Vitamin D metabolites and bone mineral density: the Multi-Ethnic Study of Atherosclerosis. Bone. 2015;78:186–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dawson-Hughes B, Harris SS, Krall EA, Dallal GE, Falconer G, Green CL. Rates of bone loss in postmenopausal women randomly assigned to one of two dosages of vitamin D. Am J Clin Nutr 1995;61:1140–1145. [DOI] [PubMed] [Google Scholar]
- 20.Kremer R, Campbell PP, Reinhardt T, Gilsanz V. Vitamin D status and its relationship to body fat, final height, and peak bone mass in young women. J Clin Endocrinol Metab 2009;94:67–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Regard JB, Zhong Z, Williams BO, Yang Y. Wnt signaling in bone development and disease: making stronger bone with Wnts. Cold Spring Harb Perspect Biol 2012. December 1;4(12):a007997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parfitt AM, Mathews CH, Brommage R, Jarnagin K, DeLuca HF. Calcitriol but no other metabolite of vitamin D is essential for normal bone growth and development in the rat. J Clin Invest 1984. February;73(2):576–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Curtis KM, Aenlle KK, Roos BA, Howard GA. 24R,25-dihydroxyvitamin D3 promotes the osteoblastic differentiation of human mesenchymal stem cells. Mol Endocrinol 2014. May;28(5):644–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ornoy A, Goodwin D, Noff D, Edelstein S. 24, 25-dihydroxyvitamin D is a metabolite of vitamin D essential for bone formation. Nature. 1978. November 30;276(5687):517–9. [DOI] [PubMed] [Google Scholar]
- 25.Bosworth CR, Levin G, Robinson-Cohen C, Hoofnagle AN, Ruzinski J, Young B, Schwartz SM, Himmelfarb J, Kestenbaum B, de Boer IH. The serum 24,25-dihydroxyvitamin D concentration, a marker of vitamin D catabolism, is reduced in chronic kidney disease. Kidney Int 2012. September;82(6):693–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Berg AH, Bhan I, Powe C et al. Acute homeostatic changes following vitamin D2 supplementation. J Endocr Soc 2017; 1: 1135–1149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ginsberg C, Zelnick LR, Block GA, Chertow GM, Chonchol M, Hoofnagle AN, Kestenbaum BR, de Boer IH. Differential Effects of Phosphate Binders on Vitamin D Metabolism in Chronic Kidney Disease. Nephrol Dial Transplant 2020. April 1;35(4):616–623 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Kidney Function declined as a decline in eGFR by of ≥30% from baseline
Data Availability Statement
The data that support the findings of this study are available from Health ABC. Restrictions apply to the availability of these data, which were used under license for this study. Data are available https://healthabc.nia.nih.gov with the permission of Health ABC.


