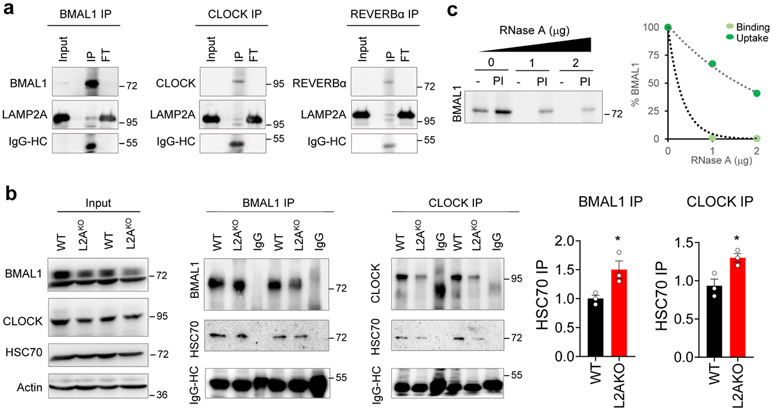
Extended Data Fig. 1. Circadian proteins display properties of bona fide CMA substrates.
a. Representative immunoblot for LAMP2A from rat liver lysosomes immunoprecipitated for BMAL1 (left), CLOCK (middle), or REVERBα (right). The heavy chain (HC) of IgG used in the immunoprecipitation is shown. Input is 5% amount used for IP. b. Representative immunoblots for HSC70 from wild-type (WT) or LAMP2A knockout (L2AKO) cell lysates immunoprecipitated for BMAL1 or CLOCK. Heavy chain (HC) for IgG used in the immunoprecipitation is shown. Input is 5% concentration used for IP. Right, quantification of HSC70 normalized by the amount of BMAL1 or CLOCK immunoprecipitated. An increase in the amount of CMA substrates bound at a given time to HSC70, similar to the one observed here for BMAL1 and CLOCK, has previously been described upon blockage of CMA. n=3 independent experiments. Individual values (b) and mean+s.e.m are shown. Unpaired two-tailed t test was used, and differences were significant for *p<0.05. c. Representative immunoblot of competition of BMAL1 lysosomal uptake by ribonuclease A (RNase A). Left: representative immunoblot. Right: effect of increasing concentrations of RNase A on binding and uptake of 20ng of BMAL1. Numerical source data, statistics and exact p values and unprocessed blots are available as source data.