FIGURE 1.
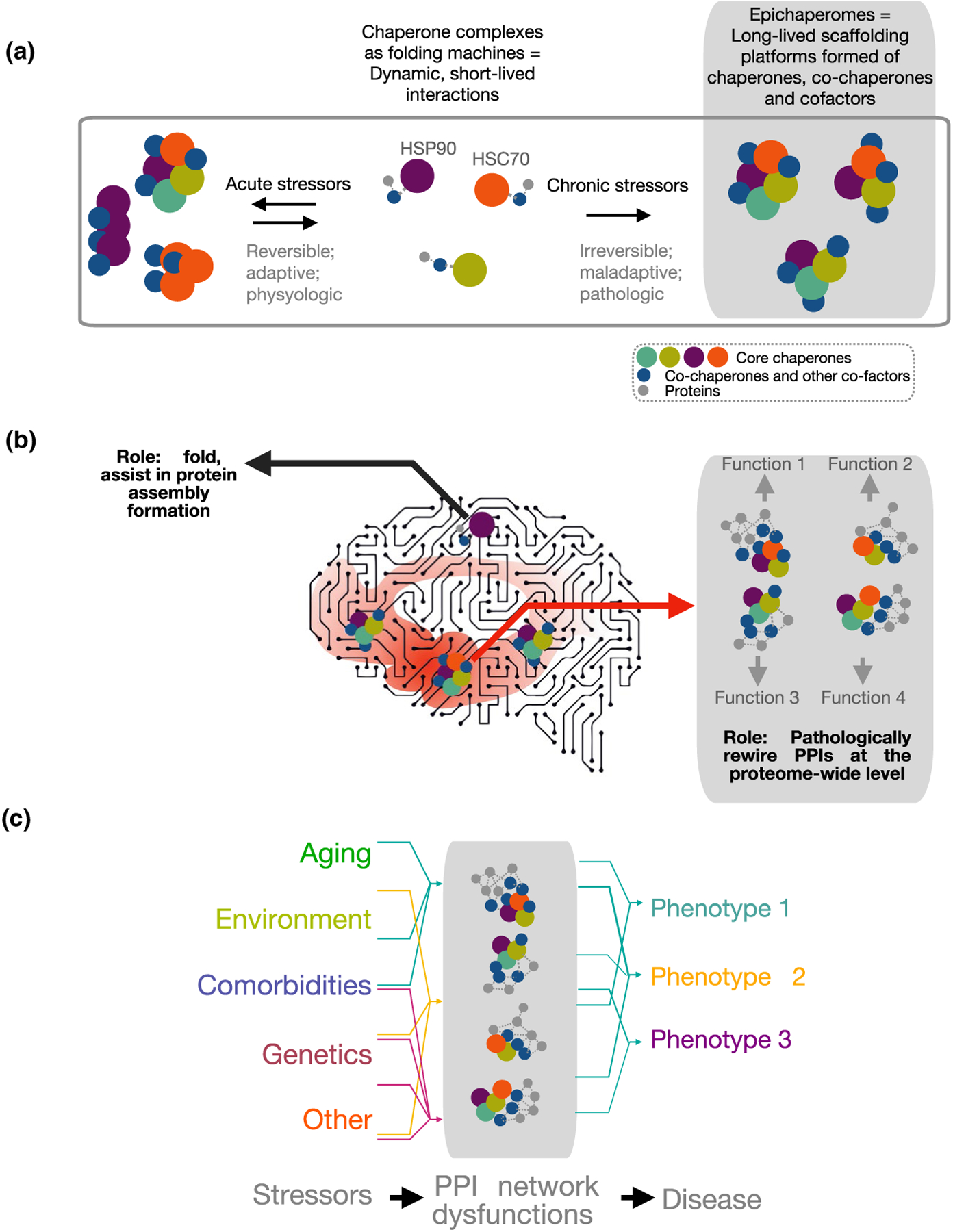
Biochemical (a) and functional (b, c) distinction between chaperones and epichaperomes. There is a fundamental structural, dynamic, and functional difference between HSP90 and HSP90 incorporated into an epichaperome. Epichaperomes are scaffolds. They rewire the connectivity and function of protein networks by remodeling how thousands of proteins interact in conditions of chronic cellular stress. Conversely, HSP90 is a chaperone. Chaperones, co-chaperones, and their complexes have defined functions as protein folders. They interact with a protein to process it through the chaperone folding cycle. Epichaperomes are long-lived oligomers of chaperome members. This differs from chaperones such as HSP90, which interact in a highly dynamic manner with one another and with client proteins on the millisecond to second timescale to make folding versus degradation decisions through transient interactions within the context of the proteostasis network. Epichaperomes are specific to cells exposed to defined stressors and/or combination of stressors. Conversely, chaperones are highly abundant and ubiquitous proteins. Epichaperome composition is stressor specific with distinct epichaperome assemblies impacting specific proteins, and in turn, protein pathways. Therefore, epichaperomes are multimeric, long-lived chaperome structures that act as scaffold for remodeling PPIs and provide a link between stressor-induced protein–protein interaction network perturbations and phenotypes. Abbreviations: HSP90, heat shock protein 90 (HSP90α and HSP90β isoforms, encoded by the HSP90AA1 and HSP90AB1 genes, respectively; HSC70, heat shock cognate 70 protein encoded by the HSPA8 gene; PPIs, protein–protein interactions
