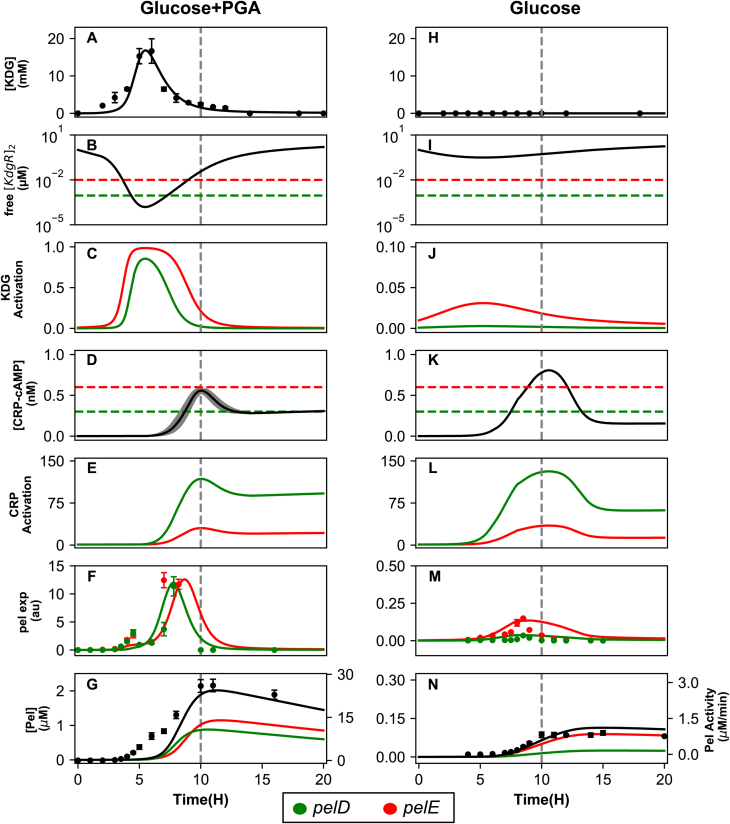
Figure 5.
Modeling of pel regulatory pathways and expression in bacteria grown in minimal medium supplemented with glucose+PGA (left column) or glucose (right). The model results are shown as solid lines, and the experimental data as dots. The transition time is shown as a dashed gray vertical line. A, KDG intracellular concentration superimposed with HPLC direct measurement data exhibiting a peak after around 6 h of growth; B, concentration of intracellular free KdgR dimer, with horizontal dashed lines showing the binding affinities of the dimer for the pelE (red) and pelD (green) promoters, indicating the thresholds of repression; C, pelE and pelD (depicted in red and green, respectively throughout the figure) activation curves based on KdgR binding, exhibiting a derepression in the middle of exponential phase due to high intracellular levels of KDG; D, intracellular concentration of the cAMP–CRP complex inferred from HPLC measurement of cAMP in the medium (Fig. 4); the gray area is the 95% confidence interval (from two biological replicates), and the dashed horizontal lines indicate the binding affinities for pelD/E promoters; E, pel activation curves based on cAMP-CRP binding, with a boost occuring at transition; F, pel expression time course based on the combined regulation by KdgR and CRP, superimposed with qRT-PCR datapoints peaking around 8 h; G, extracellular concentration of Pel enzymes, either from individual genes (red and green) or the combination of both (black), superimposed with the measurements of enzymatic activity (dots) reflecting total Pel enzymes, including those not considered in our modeling. H–N, same legends as A–G: in the absence of PGA, the dilution of KdgR during growth is sufficient to partly relieve pelE repression, whereas cAMP induces the expression of both pels close to transition. The timing and expression levels of both genes are accurately reproduced by the model in both conditions, as well as the inducing effect of PGA. CRP, cAMP receptor protein; KDG, 2-keto-3-deoxygluconate; Pel, endo-pectate lyase; PGA, polygalacturonate.