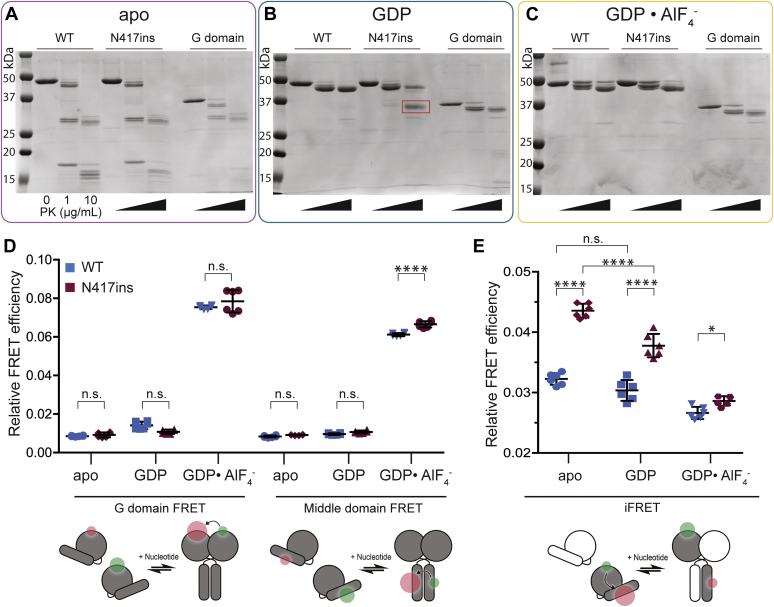
Figure 6.
Altered conformation of the monomeric ATL1 N417ins.A–C, SDS-PAGE gels show limited proteolysis reactions with increasing concentrations of proteinase K (PK) from 0 to 10 μg/ml and 2 μM ATL1 (catalytic core of ATL1 WT and N417ins; purified G domain) for 15 min on ice. Reactions were incubated either in the absence of nucleotide (A), with 2 mM GDP (B), or with GDP•AlF4− (C). The red box in (B) indicates a band of interest for the mutant and molecular weight markers are indicated on the left of each gel. D, intermolecular FRET between G domains (left) and middle domains (right) for ATL1 WT (blue) and N417ins (maroon) in the presence of indicated nucleotides (X-axis). Relative FRET efficiencies shown by symbols for three technical and two biological replicates for each condition (n = 6) with mean shown by the middle bar and SD by error bars. E, iFRET within ATL1 WT (blue) or N417ins (maroon) carried out with a 1:10 excess of unlabeled protein. Conditions and statistics are the same as in (D). Cartoons at the bottom of (D and E) represent experimental setups.