Abstract
Aberrant responses to ultraviolet (UV) light frequently lead to formation of skin lesions and activation of systemic disease in some autoimmune diseases, especially systemic lupus erythematosus. While the effects of UV light on the skin have been studied for decades, only recently have some of the mechanisms that contribute to abnormal responses to UV light in autoimmune disease patients been uncovered. This review will discuss the biology of UV in the epidermis and discuss the abnormal epidermal and inflammatory mechanisms that contribute to photosensitivity. Further research is required to fully understand how to normalize UV-mediated inflammation in autoimmune patients.
Keywords: autoimmunity, dermatomyositis, lupus, photosensitivity, ultraviolet light
INTRODUCTION
For many decades, ultraviolet (UV) light has been recognized as an inhibitor of antigen-specific cell-mediated immunity (Kripke and Fisher, 1976). Mechanisms driving UVB-mediated immunosuppression in the skin are complex and include suppression of effector and memory T cell responses (Rana et al., 2008), activation of regulatory T cells (Bruhs and Schwarz, 2017, Shreedhar et al., 1998), increase in suppressive B cell activation (Byrne and Halliday, 2005), and recruitment of activated neutrophils that produce IL-10 (Piskin et al., 2005). However, UV light is also a well-recognized trigger for skin inflammation in susceptible autoimmune patients, especially systemic and cutaneous lupus and dermatomyositis. Understanding this dichotomy will inform preventative and therapeutic avenues for individuals with autoimmune diseases and photosensitivity. This review seeks to discuss the effects of UV light on the skin and contrast these effects in healthy and autoimmune individuals with a primary focus on systemic and cutaneous lupus patients as these diseases have the most data available for effects of UV light.
EFFECTS OF UV LIGHT
Damage to nucleic acids
UV light is classified based on wavelength into UVA (320–400nm), UVB (280–320nm), and UVC (100–280nm), with shorter wavelengths possessing higher energy. Exposure to both UVA and UVB light triggers a wide range of intracellular processes that results in damage, repair, death, and inflammation with outcomes dependent on duration and intensity of exposure. When DNA absorbs UVB photons, intra-strand linkages can form photodimers including cyclobutane pyrimidine dimers (CPDs) and pyrimidine-6,4-pyrimidone photoproducts (6,4PPs). UVA exerts the majority of its cellular effects indirectly through generation of reactive oxygen species (ROS) that induce oxidation of macromolecular structures (Pattison and Davies, 2006). These oxidants damage proteins and lipids, resulting in modulation of cellular signaling pathways and membrane structures, which has detrimental effects on the cell (Batista et al., 2009, Pattison et al., 2012). Oxidative modification of DNA also promotes formation of oxidized bases such as 8-hydroxy-2’-deoxyguanosine (8-OHdG). To limit mutagenicity, the oxidized guanine must be repaired via oxyguanine glycosylase 1 (OGG1)-initiated base excision repair (Paz-Elizur et al., 2008). Intriguingly, loss of Ogg1 in the pristane-induced mouse model of systemic lupus erythematosus (SLE) results in dysregulated IFN responses and aggravated skin pathology, including alopecia. Reduced expression of OGG1 is observed in lesional skin of patients with discoid lupus (Tumurkhuu et al., 2020) and 8-OHdG is abundant in UV-induced lupus skin lesions (Gehrke N. et al., 2013), suggesting a role for 8-OHdG signaling in lesion development. Intriguingly, a role for UVB-mediated damage of RNA in generating inflammatory responses has also been reported. UV-induced changes in non-coding RNAs, which are secreted by keratinocytes after UV exposure, facilitate inflammatory signaling and release of TNFα and IL-6 through triggering of TLR3(Bernard et al., 2012).
Cell Death
Cells can initiate apoptosis as a protective mechanism when there is irreparable DNA damage. Apoptosis is a form of programmed cell death that is generally mediated by caspases and occurs through either the intrinsic pathway (mediated by mitochondrial dysfunction) or the extrinsic pathway (mediated by activation of external death receptors). During apoptosis, cells undergo morphological changes, but the plasma membrane remains intact to prevent release of inflammatory cellular contents, making this an immunologically silent process (Elmore, 2007). Accumulation of apoptotic cells has been noted in the epidermis of SLE patients after UV exposure (Kuhn et al., 2006) which may be secondary to increased cell death and/or decreased clearance. Reduced clearance of apoptotic cells is associated with decreased levels of serum complement proteins C1q, C4, and C3 (Bijl et al., 2006, Ren et al., 2003). Importantly, photosensitivity is more common among patients with deficiencies of C4A (Sturfelt et al., 1990) and C2 (Chen et al., 2015). Other forms of cell death have been reported in cutaneous lupus. Necroptotic cell death has been reported in interface dermatitis, such as CLE and dermatomyositis, but whether this is the primary method of cell death after UVB exposure is unknown(Lauffer et al., 2018). Increased caspase-1 and inflammasome mediators have also been noted in CLE lesions, but whether caspase-1 mediated pyroptosis plays a role after UVB also requires further investigation(Liu et al., 2017).
Autoantigens
UVB exposure induces translocation of intracellular antigens including Ro/SSA and La/SSB to the surface of apoptotic keratinocytes, rendering cells susceptible to being bound by circulating autoantibodies (Furukawa et al., 1990, Jones, 1992, Lawley et al., 2000, Wang et al., 1999). These autoantigens tend to cluster in close proximity to sites of increased ROS generation, leaving them vulnerable to oxidative modifications that further enhance their immunogenicity (Casciola-Rosen et al., 1994). The presence of anti-Ro and anti-La autoantibodies as well as increased expression of Ro/SSA and La/SSB on keratinocytes correlate with patient photosensitivity (Ioannides et al., 2000, McHugh et al., 1990, Menéndez et al., 2013, Mond et al., 1989). UVB can also increase autoantibody binding to other autoantigens including Sm, RNP, Ku, and ribosomal-P (Caricchio et al., 2003, Golan et al., 1992, Shi et al., 2015), and this is associated with photosensitivity in lupus patients (Fredi et al., 2014, Gerli et al., 2002, Shi et al., 2015). Importantly, deposition of antibodies at the dermal-epidermal junction can be induced by UV stimulation (Fabre et al., 1991). These antibodies, when bound to antigen, form immune complexes that can amplify the inflammatory response through a variety of mechanisms. Autoantibodies in complex with RNA or DNA fragments can be internalized by FcγRII on pDCs resulting in activation of endosomal Toll-like receptor (TLR)7/9 and production of interferon (IFN)-α (Barrat et al., 2005, Means et al., 2005, Meller et al., 2005). Immune complexes can also stimulate inflammasome activation (Shin et al., 2012, Shin et al., 2013) and B cell expansion (Berggren et al., 2017), which may further perpetuate the cycle of inflammation in the skin following UV exposure in predisposed individuals.
Langerhans cells
UV exposure results in the activation of epidermal growth factor receptor (EGFR), a transmembrane protein involved in regulating proliferation, differentiation, and survival of keratinocytes. EGFR activation enhances keratinocyte replication leading to epidermal hyperplasia that protects against subsequent UV-induced skin injury (El-Abaseri et al., 2006). Langerhans cells (LCs), a population of antigen-presenting cells in the epidermis, activate EGFR via the actions of LC-expressed a disintegrin and metalloprotease 17 (ADAM17) (Shipman et al., 2018), which limits UV-induced keratinocyte apoptosis. LCs play a further role in limiting skin injury following UV radiation through their phagocytosis of apoptotic keratinocytes (Hatakeyama et al., 2017). Importantly, reduced numbers of LCs are found in SLE skin compared to healthy control skin and this coincides with reduced epidermal EGFR phosphorylation (Shipman et al., 2018).
Cytokines
UV exposure influences cytokine production in a highly context-dependent manner. UV induces the production of inflammatory cytokines such as type I IFN, TNFα, IL-6 and IL-1β, and these can feed forward to prime for additional inflammatory responses to UVB(Bashir Muhammad M. et al., 2009, Stannard et al., 2017a). In WT mouse skin, exposure to UVB radiation enhances stimulator of interferon genes (STING)-dependent production of type I IFNs in a bimodal fashion, with early production likely by keratinocytes (Skopelja-Gardner et al., 2020, Stannard et al., 2017b) and later production by infiltrating immune cells, including inflammatory monocytes (Sontheimer et al., 2017). Wild-type mice with type I IFN receptor (IFNAR)-knockout displayed decreased inflammation after a single exposure of UVB but more severe skin inflammation after multiple doses of UVB, suggesting that type I IFNs could play a protective role in healthy skin (Sontheimer et al., 2017). This suppressive effect may occur via IFN-induced upregulation of the RNA-binding protein tristetrapolin, which limits expression of pro-inflammatory genes such as TNFα and IL-6 (Sauer et al., 2006).
Cell recruitment
Neutrophils are considered first responders of the immune system and, as such, are among the first cells to be recruited into the skin following UVB irradiation (Cela et al., 2015, Fisher et al., 2001, Hawk et al., 1988, Schornagel et al., 2004, Sontheimer et al., 2017, Takeuchi et al., 2010). In healthy skin, these responding neutrophils express high levels of IL-10 that contributes to an immunosuppressive environment (Piskin et al., 2005). Intriguingly, neutrophil infiltration after UVB exposure is significantly reduced in the skin of patients with photosensitive disorders such as polymorphous light eruption, and this likely limits immunosuppression (Schornagel et al., 2004). Other innate cell populations including mast cells, via keratinocyte-derived IL-15 and CCL5 (Van Nguyen et al., 2011) and plasmacytoid dendritic cells (pDCs), via dermal fibroblast production of the chemoattractant chemerin (Yin et al., 2014) are also recruited after UV exposure
Human skin has a large population of resident T cells that provides surveillance and repair functions following exposure to UV light. Specifically, UVB radiation induces release of ATP from keratinocytes (Takai et al., 2011) that can activate these skin-resident T cells and increase their production of IL-17 (MacLeod et al., 2014). This upregulates keratinocyte expression of two DNA damage associated genes, TNF related weak inducer of apoptosis (TWEAK) and Growth arrest and DNA damage associated gene 45 (GADD45) (Hildesheim et al., 2002, Sabour Alaoui et al., 2012), and thus, limits DNA damage in the keratinocytes (MacLeod et al., 2014). Local type I IFN production triggered by UV light enhances production of Th1-associated chemokines CXCL9, CXCL10, and CXCL11 which supports T cell recruitment into the skin (Di Nuzzo et al., 1996, Meller et al., 2005). This influx of CD4+ T cells is followed by induction of regulatory T cells (Tregs) with immunosuppressive functions (Bruhs and Schwarz, 2017).
B cells also play a role in UV-induced immunosuppression in healthy skin. Specifically, UVB irradiation activates regulatory B cells in skin-draining lymph nodes, potentially via IL-10, that can inhibit dendritic cell-mediated activation of T cell immunity (Byrne and Halliday, 2005). Intriguingly, new roles for skin-associated B cells in both driving and suppressing cutaneous inflammation have recently been identified (Debes and McGettigan, 2019). Elevated numbers of B cells have been observed in lesional DLE skin relative to controls (Hussein et al., 2008, O’Brien et al., 2017, Wouters et al., 2004, Xie et al., 2011); however, the disease specific functions of these B cells have yet to be determined. As such, it is not currently known if differential activation of regulatory or inflammatory B cells by UV is involved in development of autoimmune photosensitive responses.
UV-induced STING activation
UVB-induced activation of nucleic acid sensing results in UVB-mediated IFN production through activation of the cyclic GMP-AMP synthase (cGAS)-STING pathways. In vivo, acute upregulation of type I IFNs in the skin is dependent on cGAS(Skopelja-Gardner et al., 2020). Upon binding to cytosolic DNA, cGAS enzymatically generates cGAMP, an upstream STING agonist (Ablasser et al., 2013, Li X. et al., 2013). Following activation, STING induces tank binding kinase 1 (TBK1)-driven phosphorylation of interferon regulatory factor 3 (IFR3) and NF-kB, which activates type I IFN and proinflammatory cytokine expression (Hopfner and Hornung, 2020) (Abe and Barber, 2014, Fitzgerald et al., 2003, Motwani et al., 2019). Sources of cytosolic DNA that activate cGAS-STING signaling originate from viral and bacterial infection, chromosomal damage and micronuclei, and mitochondrial DNA leakage (Li X. D. et al., 2013), (Prantner et al., 2010), (Watson et al., 2015), (Harding et al., 2017), (Mackenzie et al., 2017), (White et al., 2014), (Rongvaux et al., 2014). Moreover, UV-induced DNA damage generates more immunostimulatory forms of nucleic acids including 8-OHdG, which demonstrates resistance to three prime repair exonuclease 1 (TREX-1)-mediated degradation, resulting in the cytosolic accumulation and consequent STING-dependent immune recognition (Gehrke N. et al., 2013). Of note, the oxidized base 8-OHdG is abundant in the epidermis of UV-induced lupus lesions, where it colocalizes with the IFN-induced gene MxA (Gehrke N. et al., 2013), suggesting UV-induced modification of DNA is one mechanism by which IFNs are upregulated in lupus skin. Further, UV irradiation-induced apoptosis may result in Bax/Bak-mediated mitochondrial DNA release into the cytosol that also activates STING-dependent type I IFN expression (White et al., 2014), (Rongvaux et al., 2014), (Barber, 2015). UV-induced apoptotic signaling also depletes ULK1, a negative STING regulator, which upregulates STING-dependent IRF-3 phosphorylation (Kemp et al., 2015). In sum, UVB induced cellular changes result in type I IFN production through cGAS-STING activation.
DYSREGULATED ULTRAVIOLET RESPONSES IN AUTOIMMUNE PATIENTS
While UVB exposure suppresses immune responses in healthy individuals, it is a well-described trigger of skin manifestations in numerous autoimmune diseases including systemic and cutaneous lupus erythematosus (SLE, CLE), dermatomyositis (DM), and Sjogren’s syndrome (SS). In lupus, it’s been reported that up to 93% of patients experience photosensitivity depending on underlying disease pathology (Hasan et al., 1997, Kuhn and Landmann, 2014, Sanders et al., 2003), while up to 50% of DM patients are reported to have photosensitive skin disease (Dourmishev et al., 2004). Exact mechanisms governing UV-mediated cutaneous inflammation in these diseases remain poorly described; ongoing studies to elucidate such mechanisms are reviewed below.
Systemic lupus erythematosus
SLE is a heterogeneous autoimmune disorder characterized by a high rate of sensitivity to UV light whereby patients develop skin lesions, termed cutaneous lupus erythematosus (CLE), following UV exposure. While the precise mechanisms leading to UVB mediated inflammation are poorly understood, most evidence points to overproduction of inflammatory mediators and increased cell recruitment as likely contributors.
UV light triggers production of several pro-inflammatory cytokines, including TNFα, IL-6, and IL-1α/β (Avalos-Díaz et al., 1999, Bashir M. M. et al., 2009, Brink et al., 2000, Clingen et al., 2001, Köck et al., 1990, Takashima and Bergstresser, 1996, Yarosh et al., 2000), that contribute to cutaneous inflammation provoked during sun-induced lupus flares. These cytokines can, in turn, promote production of inflammatory chemokines such as CCL5, CCL20, CCL22, and CXCL8 by epidermal keratinocytes and enhance leukocyte recruitment into the skin (Meller et al., 2005). Supporting a role for UV-induced injury in the inflammatory phenotype of cutaneous lupus, CCL5 and CXCL8 are among the most differentially regulated chemokines in CLE (Meller et al., 2005). CCL27, a skin-specific chemokine that is produced in response to TNFα and IL-1β stimulation increases recruitment of memory T cells into the skin that can release large amounts of IFN-γ and further perpetuate inflammation (Homey et al., 2002, Meller et al., 2005, Morales et al., 1999).
Murine models have identified potential mechanisms for UVB-induced skin inflammation in lupus. Monocytes are a source of type I IFN production after UV exposure (Sontheimer et al., 2017), and in the MRL-Faslpr mouse model of lupus, UVB irradiation was shown to increase keratinocyte production of CSF-1, which was necessary for macrophage infiltration and CLE-like lesion development (Menke et al., 2008). These data suggest monocytes as important contributors to skin inflammation in UV-mediated injury in mice. Human studies have validated the importance of monocytic inflammation to CLE lesion development as a monocytic signature is noted in lesions of CLE patients (Berthier et al., 2019), and type I IFN-stimulated gene expression is correlated with infiltration of monocytes in the UV-exposed skin of lupus patients (Reefman et al., 2008).
Like monocytes, neutrophils also home to the skin early in UV-mediated inflammation in lupus. A recent study using a murine model of CLE with deletion of PD-1H revealed neutrophils in the skin before onset of lesions, suggesting neutrophils as important early drivers of CLE lesions (Han et al., 2019). In addition, low density granulocytes, cells which produce excess neutrophil extracellular traps (NETs), are associated with CLE lesions, and NETs have been found in CLE lesions (Denny et al., 2010, Villanueva et al., 2011). Exposure to UV light can also induce NETosis, which may serve as a link between UV exposure and CLE (Neubert et al., 2019). Indeed, NETs have now been identified to activate cGAS, potentially linking NETs with type IFN production in the skin(Apel et al., 2021). Fascinatingly, skin exposure to high doses of UVB light also stimulates neutrophil migration into the kidneys where they contribute to renal inflammation, injury, and type I IFN signatures (Skopelja-Gardner et al., 2021), suggesting a possible mechanistic link between photosensitivity and systemic disease flares. Besides inducing NETosis, UV light exposure has also been shown to recruit plasmacytoid dendritic cells (pDCs) to the skin of lupus-prone mice and to a greater extent in SLE patients vs. healthy controls. (Yin et al., 2014, Zahn et al., 2014), possibly secondary to a higher expression of chemerin in SLE patients. (Vermi et al., 2005).
Mast cells are also dysregulated in CLE; the numbers are elevated in sun-exposed vs. sun-protected CLE skin (Van Nguyen et al., 2011). Mast cells may contribute to cutaneous inflammation via production of matrix metalloproteinases (MMPs). Indeed, CLE lesions exhibit elevated expression of activated MMPs, including MMP-1 and MMP-9, with levels of active MMP-9 correlating with cutaneous disease severity (Ertugrul et al., 2018, Van Nguyen et al., 2011). Further investigation into the role of UVB-recruited mast cells and their secreted MMPs is needed to clarify their roles in autoimmune photosensitivity.
UV exposure has been shown to recruit T cells to the dermoepidermal junction in lesional skin of lupus patients (Peter Kind and Plewig, 1993) and subtypes of CLE demonstrate a T cell signature (Berthier et al., 2019, Solé et al., 2016). Tregs, which suppress inflammation in response to UV light, are decreased in CLE lesions (Franz et al., 2007). In the NZM2328 murine model of lupus, UVB exposure leads to decreased Treg differentiation and increased effector T cell activation in skin draining lymph nodes in a type I IFN dependent fashion (Wolf et al., 2019). Further, human UV photoprovocation studies have identified increased expression of genes related to antigen presentation in the skin of CLE patients, which would also result in T cell activation in situ (Katayama et al., 2019). Recent early phase trials have shown biomarker improvement with restoration of Treg suppressive capacity in CLE, including adoptive transfer of autologous polyclonal Tregs (Dall’Era et al., 2019) and use of low-dose IL-2 to expand Tregs (He et al., 2020), but whether Treg expansion alters photosensitive responses is unknown.
The epidermis itself is abnormal in SLE patients and also contributes to abnormal UV responses in SLE patients. Interferon response genes are more highly expressed in non-lesional lupus keratinocytes (Der et al., 2019, Psarras et al., 2020, Stannard et al., 2017b) compared to healthy controls and this chronic upregulation may trigger a more inflammatory response to UV radiation. In addition, SLE keratinocytes exhibit a more robust response to type I IFN stimulation, which could lead to this enhanced UV response with lower doses of IFN exposure(Tsoi et al., 2019). Following UVB stimulation, SLE keratinocytes secrete more IFN-κ, a type I IFN produced by keratinocytes, compared to healthy controls (Stannard et al., 2017b) and this increases keratinocyte apoptosis following UV radiation (Sarkar et al., 2018). Conditioned media from UVB irradiated SLE keratinocytes stimulates dendritic cell activation in an IFN-dependent manner, suggesting epithelial-derived IFN-κ primes SLE skin for heightened UVB-induced inflammation (Sarkar et al., 2018). Similar photosensitization has been shown with TLR3 priming to increase IFN production (De Groof et al., 2020). Intriguingly, SLE-risk polymorphisms in RNase H2, which impede ribonucleotide excision repair, can increase CPD formation and the IFN-stimulatory capacity of the damaged DNA(Gunther et al., 2015). Together, these data support a pathogenic role for chronic type I IFNs in lupus skin following UVB exposure.
Dermatomyositis
Dermatomyositis (DM) is an inflammatory myositis characterized by symmetric, proximal muscle weakness, and cutaneous manifestations often distributed in UV-exposed areas, suggesting the importance of UV light to disease pathogenesis. As in SLE, the minimal amount of UVB light needed to induce erythema in patients with DM is decreased (Dourmishev et al., 2004), and DM lesional skin also exhibits increased apoptotic keratinocytes(Pablos et al., 1999). Further, in a retrospective study of geographic UV intensity and development of DM, women who lived in locations with higher UV exposure were more likely to develop DM (Parks et al., 2020). Consistent with this, self-reported sun exposure is associated with DM flare (Mamyrova et al., 2017). Another study identified a correlation between levels of UV exposure and expression of Mi-2 autoantibodies (Okada et al., 2003). Intriguingly, treatment of keratinocyte cell lines with UV light has been shown to increase Mi-2 protein expression, which may provide a mechanistic link between UV exposure and DM disease flares (Burd et al., 2008). Importantly, DM patients also exhibit a strong type I IFN gene signature, possibly linked to production of specific autoantibodies such as anti-MDA5, and production of IFN-κ and IFN-β (Cassius et al., 2020, Tsoi et al., 2020, Turnier et al., 2020, Wong et al., 2012), but whether this contributes to photosensitivity in the same manner as SLE remains to be determined. It is unknown whether DM patients exhibit other abnormalities in cell death or cytokine production to UVB light.
Sjögren’s Syndrome
Sjögren’s syndrome (SS) is an autoimmune disorder characterized by exocrine glandular features such as dry eyes, mouth, and decreased salivary gland function, as well as extraglandular manifestations. Cutaneous manifestations of SS are diverse and include annular erythema (AE), a non-scarring erythematous lesion associated with anti-Ro antibodies which resembles but is distinct from subacute cutaneous lupus. In one study of primary SS patients with AE, 100% of patients reported photosensitivity, and 93% of patients reported cutaneous flares during summer months (Brito-Zerón et al., 2014). In another study of SS patients, patients were exposed to UV light and epidermal changes were noted, and 11 of the 14 patients tested demonstrated photosensitive responses (Tsukazaki et al., 2002). While such studies and clinical experience have pointed to UV light as a trigger of SS disease manifestations, the exact mechanisms are understudied and provide an interesting and necessary avenue for further investigation.
SUMMARY
Accumulating evidence demonstrates that the response to UV light is distorted in autoimmune disease patients with photosensitivity. Skewing of inflammatory responses, inhibition of regulatory responses and chronic type I IFN exposure all likely contribute. Further investigations into mechanisms of photosensitivity in autoimmune disease patients offers the opportunity to develop preventive therapies.
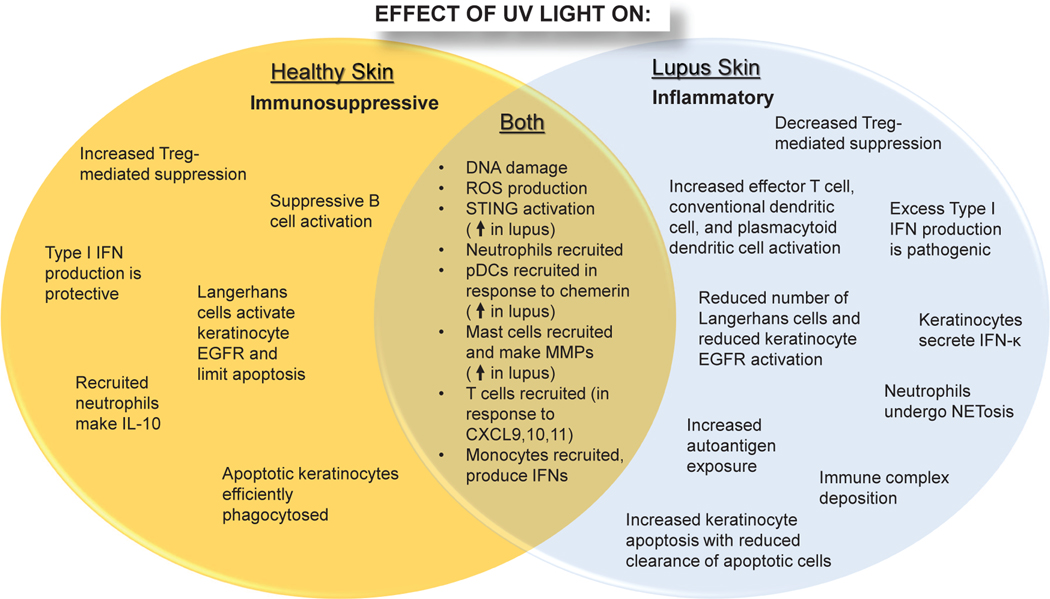
Figure 1: Overview of differential effects of UV light on healthy vs. lupus skin.
In healthy skin (left), UV light generates an immunosuppressive environment characterized by efficient clearance of apoptotic cells, immune cell activation, and secretion of protective/suppressive cytokines including type I interferons (IFNs) and IL-10. In lupus skin, UV light exposure is inflammatory secondary to increased immune cell infiltration, inhibition of negative regulatory mechanisms, and amplified production of type I IFNs that enhance keratinocyte apoptosis. Apoptotic cells are not efficiently cleared resulting in increased autoantigen exposure, immune complex formation, and lesion development.
Acknowledgements
This work was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under award numbers R01-AR071384 (J.M.K.), K24-AR076975 (J.M.K), P30-AR075043 (J.M.K.), F31 AR077988 (SNE), the National Institute of Allergy and Infectious Diseases under award T32-AI007413 (SNE), The National Institute of General Medical Sciences under award T32-GM007863 (MPM), the Lupus Research Alliance (J.M.K.), the A. Alfred Taubman Medical Research Institute (J.M.K.), the Parfet Emerging Scholar Award (J.M.K.), and the U-M Undergraduate Research Opportunities Program (JM).
Footnotes
Data Availability: No large-scale datasets were generated in this manuscript.
Conflict of Interest: JMK has received Grant support from Q32 Bio, Celgene/BMS and Janssen. JMK has served on advisory boards for AstraZeneca, Eli Lilly, GlaxoSmithKline, Bristol Myers Squibb, Avion Pharmaceuticals, Provention Bio, Aurinia Pharmaceuticals, Ventus Therapeutics, and Boehringer Ingelheim. All other authors have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abe T, Barber GN. Cytosolic-DNA-mediated, STING-dependent proinflammatory gene induction necessitates canonical NF-kappaB activation through TBK1. J Virol 2014;88(10):5328–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ablasser A, Goldeck M, Cavlar T, Deimling T, Witte G, Rohl I, et al. cGAS produces a 2’−5’-linked cyclic dinucleotide second messenger that activates STING. Nature 2013;498(7454):380–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apel F, Andreeva L, Knackstedt LS, Streeck R, Frese CK, Goosmann C, et al. The cytosolic DNA sensor cGAS recognizes neutrophil extracellular traps. Sci Signal 2021;14(673). [DOI] [PubMed] [Google Scholar]
- Avalos-Díaz E, Alvarado-Flores E, Herrera-Esparza R. UV-A irradiation induces transcription of IL-6 and TNF alpha genes in human keratinocytes and dermal fibroblasts. Revue du rhumatisme (English ed) 1999;66(1):13–9. [PubMed] [Google Scholar]
- Barber GN. STING: infection, inflammation and cancer. Nat Rev Immunol 2015;15(12):760–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrat FJ, Meeker T, Gregorio J, Chan JH, Uematsu S, Akira S, et al. Nucleic acids of mammalian origin can act as endogenous ligands for Toll-like receptors and may promote systemic lupus erythematosus. The Journal of experimental medicine 2005;202(8):1131–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashir MM, Sharma MR, Werth VP. UVB and proinflammatory cytokines synergistically activate TNF-alpha production in keratinocytes through enhanced gene transcription. The Journal of investigative dermatology 2009;129(4):994–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batista LFZ, Kaina B, Meneghini R, Menck CFM. How DNA lesions are turned into powerful killing structures: insights from UV-induced apoptosis. Mutation research 2009;681(2–3):197–208. [DOI] [PubMed] [Google Scholar]
- Berggren O, Hagberg N, Alexsson A, Weber G, Ronnblom L, Eloranta ML. Plasmacytoid dendritic cells and RNA-containing immune complexes drive expansion of peripheral B cell subsets with an SLE-like phenotype. PLoS One 2017;12(8):e0183946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard JJ, Cowing-Zitron C, Nakatsuji T, Muehleisen B, Muto J, Borkowski AW, et al. Ultraviolet radiation damages self noncoding RNA and is detected by TLR3. Nature medicine 2012;18(8):1286–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthier CC, Tsoi LC, Reed TJ, Stannard JN, Myers EM, Namas R, et al. Molecular Profiling of Cutaneous Lupus Lesions Identifies Subgroups Distinct from Clinical Phenotypes. J Clin Med 2019;8(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bijl M, Reefman E, Horst G, Limburg PC, Kallenberg CG. Reduced uptake of apoptotic cells by macrophages in systemic lupus erythematosus: correlates with decreased serum levels of complement. Annals of the rheumatic diseases 2006;65(1):57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brink N, Szamel M, Young AR, Wittern KP, Bergemann J. Comparative quantification of IL-1beta, IL-10, IL-10r, TNFalpha and IL-7 mRNA levels in UV-irradiated human skin in vivo. Inflammation research : official journal of the European Histamine Research Society [et al. ] 2000;49(6):290–6. [DOI] [PubMed] [Google Scholar]
- Brito-Zerón P, Retamozo S, Akasbi M, Gandía M, Perez-De-Lis M, Soto-Cardenas MJ, et al. Annular erythema in primary Sjogren’s syndrome: description of 43 non-Asian cases. Lupus 2014;23(2):166–75. [DOI] [PubMed] [Google Scholar]
- Bruhs A, Schwarz T. Ultraviolet Radiation-Induced Immunosuppression: Induction of Regulatory T Cells. Methods in molecular biology (Clifton, NJ 2017;1559:63–73. [DOI] [PubMed] [Google Scholar]
- Burd CJ, Kinyamu HK, Miller FW, Archer TK. UV radiation regulates Mi-2 through protein translation and stability. J Biol Chem 2008;283(50):34976–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne SN, Halliday GM. B cells activated in lymph nodes in response to ultraviolet irradiation or by interleukin-10 inhibit dendritic cell induction of immunity. J Invest Dermatol 2005;124(3):570–8. [DOI] [PubMed] [Google Scholar]
- Caricchio R, McPhie L, Cohen PL. Ultraviolet B radiation-induced cell death: critical role of ultraviolet dose in inflammation and lupus autoantigen redistribution. Journal of immunology (Baltimore, Md : 1950) 2003;171(11):5778–86. [DOI] [PubMed] [Google Scholar]
- Casciola-Rosen LA, Anhalt G, Rosen A. Autoantigens targeted in systemic lupus erythematosus are clustered in two populations of surface structures on apoptotic keratinocytes. The Journal of experimental medicine 1994;179(4):1317–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassius C, Amode R, Delord M, Battistella M, Poirot J, How-Kit A, et al. MDA5(+) Dermatomyositis Is Associated with Stronger Skin Type I Interferon Transcriptomic Signature with Upregulation of IFN-κ Transcript. J Invest Dermatol 2020;140(6):1276–9.e7. [DOI] [PubMed] [Google Scholar]
- Cela EM, Friedrich A, Paz ML, Vanzulli SI, Leoni J, Gonzalez Maglio DH. Time-course study of different innate immune mediators produced by UV-irradiated skin: comparative effects of short and daily versus a single harmful UV exposure. Immunology 2015;145(1):82–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HH, Tsai LJ, Lee KR, Chen YM, Hung WT, Chen DY. Genetic association of complement component 2 polymorphism with systemic lupus erythematosus. Tissue antigens 2015;86(2):122–33. [DOI] [PubMed] [Google Scholar]
- Clingen PH, Berneburg M, Petit-Frère C, Woollons A, Lowe JE, Arlett CF, et al. Contrasting effects of an ultraviolet B and an ultraviolet A tanning lamp on interleukin-6, tumour necrosis factor-alpha and intercellular adhesion molecule-1 expression. The British journal of dermatology 2001;145(1):54–62. [DOI] [PubMed] [Google Scholar]
- Dall’Era M, Pauli ML, Remedios K, Taravati K, Sandova PM, Putnam AL, et al. Adoptive Treg Cell Therapy in a Patient With Systemic Lupus Erythematosus. Arthritis Rheumatol 2019;71(3):431–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Groof A, Ducreux J, Vidal-Bralo L, Tyteca D, Galant C, Marot L, et al. Toll-like receptor 3 increases antigen-presenting cell responses to a pro-apoptotic stimulus, yet does not contribute to systemic lupus erythematosus genetic susceptibility. Clinical and experimental rheumatology 2020;38(5):881–90. [PubMed] [Google Scholar]
- Debes GF, McGettigan SE. Skin-Associated B Cells in Health and Inflammation. Journal of immunology (Baltimore, Md : 1950) 2019;202(6):1659–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denny MF, Yalavarthi S, Zhao W, Thacker SG, Anderson M, Sandy AR, et al. A distinct subset of proinflammatory neutrophils isolated from patients with systemic lupus erythematosus induces vascular damage and synthesizes type I IFNs. J Immunol 2010;184(6):3284–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Der E, Suryawanshi H, Morozov P, Kustagi M, Goilav B, Ranabothu S, et al. Tubular cell and keratinocyte single-cell transcriptomics applied to lupus nephritis reveal type I IFN and fibrosis relevant pathways. Nature immunology 2019;20(7):915–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Nuzzo S, de Rie MA, van der Loos CM, Bos JD, Teunissen MB. Solar-simulated ultraviolet irradiation induces selective influx of CD4+ T lymphocytes in normal human skin. Photochemistry and photobiology 1996;64(6):988–93. [DOI] [PubMed] [Google Scholar]
- Dourmishev L, Meffert H, Piazena H. Dermatomyositis: comparative studies of cutaneous photosensitivity in lupus erythematosus and normal subjects. Photodermatol Photoimmunol Photomed 2004;20(5):230–4. [DOI] [PubMed] [Google Scholar]
- El-Abaseri TB, Putta S, Hansen LA. Ultraviolet irradiation induces keratinocyte proliferation and epidermal hyperplasia through the activation of the epidermal growth factor receptor. Carcinogenesis 2006;27(2):225–31. [DOI] [PubMed] [Google Scholar]
- Elmore S. Apoptosis: a review of programmed cell death. Toxicologic pathology 2007;35(4):495–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ertugrul G, Keles D, Oktay G, Aktan S. Matrix metalloproteinase-2 and −9 activity levels increase in cutaneous lupus erythematosus lesions and correlate with disease severity. Archives of dermatological research 2018;310(2):173–9. [DOI] [PubMed] [Google Scholar]
- Fabre VC, Lear S, Reichlin M, Hodge SJ, Callen JP. Twenty percent of biopsy specimens from sun-exposed skin of normal young adults demonstrate positive immunofluorescence. Arch Dermatol 1991;127(7):1006–11. [DOI] [PubMed] [Google Scholar]
- Fisher GJ, Choi HC, Bata-Csorgo Z, Shao Y, Datta S, Wang ZQ, et al. Ultraviolet irradiation increases matrix metalloproteinase-8 protein in human skin in vivo. The Journal of investigative dermatology 2001;117(2):219–26. [DOI] [PubMed] [Google Scholar]
- Fitzgerald KA, McWhirter SM, Faia KL, Rowe DC, Latz E, Golenbock DT, et al. IKKepsilon and TBK1 are essential components of the IRF3 signaling pathway. Nat Immunol 2003;4(5):491–6. [DOI] [PubMed] [Google Scholar]
- Franz B, Fritzsching B, Riehl A, Oberle N, Klemke CD, Sykora J, et al. Low number of regulatory T cells in skin lesions of patients with cutaneous lupus erythematosus. Arthritis Rheum 2007;56(6):1910–20. [DOI] [PubMed] [Google Scholar]
- Fredi M, Cavazzana I, Quinzanini M, Taraborelli M, Cartella S, Tincani A, et al. Rare autoantibodies to cellular antigens in systemic lupus erythematosus. Lupus 2014;23(7):672–7. [DOI] [PubMed] [Google Scholar]
- Furukawa F, Kashihara-Sawami M, Lyons MB, Norris DA. Binding of antibodies to the extractable nuclear antigens SS-A/Ro and SS-B/La is induced on the surface of human keratinocytes by ultraviolet light (UVL): implications for the pathogenesis of photosensitive cutaneous lupus. The Journal of investigative dermatology 1990;94(1):77–85. [DOI] [PubMed] [Google Scholar]
- Gehrke N, Mertens C, Zillinger T, Wenzel J, Bald T, Zahn S, et al. Oxidative damage of DNA confers resistance to cytosolic nuclease TREX1 degradation and potentiates STING-dependent immune sensing. Immunity 2013;39(3):482–95. [DOI] [PubMed] [Google Scholar]
- Gerli R, Caponi L, Tincani A, Scorza R, Sabbadini MG, Danieli MG, et al. Clinical and serological associations of ribosomal P autoantibodies in systemic lupus erythematosus: prospective evaluation in a large cohort of Italian patients. Rheumatology (Oxford, England) 2002;41(12):1357–66. [DOI] [PubMed] [Google Scholar]
- Golan TD, Elkon KB, Gharavi AE, Krueger JG. Enhanced membrane binding of autoantibodies to cultured keratinocytes of systemic lupus erythematosus patients after ultraviolet B/ultraviolet A irradiation. The Journal of clinical investigation 1992;90(3):1067–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunther C, Kind B, Reijns MA, Berndt N, Martinez-Bueno M, Wolf C, et al. Defective removal of ribonucleotides from DNA promotes systemic autoimmunity. J Clin Invest 2015;125(1):413–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X, Vesely MD, Yang W, Sanmamed MF, Badri T, Alawa J, et al. PD-1H (VISTA)-mediated suppression of autoimmunity in systemic and cutaneous lupus erythematosus. Sci Transl Med 2019;11(522). [DOI] [PubMed] [Google Scholar]
- Harding SM, Benci JL, Irianto J, Discher DE, Minn AJ, Greenberg RA. Mitotic progression following DNA damage enables pattern recognition within micronuclei. Nature 2017;548(7668):466–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan T, Nyberg F, Stephansson E, Puska P, Hakkinen M, Sarna S, et al. Photosensitivity in lupus erythematosus, UV photoprovocation results compared with history of photosensitivity and clinical findings. Br J Dermatol 1997;136(5):699–705. [PubMed] [Google Scholar]
- Hatakeyama M, Fukunaga A, Washio K, Taguchi K, Oda Y, Ogura K, et al. Anti-Inflammatory Role of Langerhans Cells and Apoptotic Keratinocytes in Ultraviolet-B-Induced Cutaneous Inflammation. Journal of immunology (Baltimore, Md : 1950) 2017;199(8):2937–47. [DOI] [PubMed] [Google Scholar]
- Hawk JL, Murphy GM, Holden CA. The presence of neutrophils in human cutaneous ultraviolet-B inflammation. The British journal of dermatology 1988;118(1):27–30. [DOI] [PubMed] [Google Scholar]
- He J, Zhang R, Shao M, Zhao X, Miao M, Chen J, et al. Efficacy and safety of low-dose IL-2 in the treatment of systemic lupus erythematosus: a randomised, double-blind, placebo-controlled trial. Ann Rheum Dis 2020;79(1):141–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildesheim J, Bulavin DV, Anver MR, Alvord WG, Hollander MC, Vardanian L, et al. Gadd45a protects against UV irradiation-induced skin tumors, and promotes apoptosis and stress signaling via MAPK and p53. Cancer research 2002;62(24):7305–15. [PubMed] [Google Scholar]
- Homey B, Alenius H, Müller A, Soto H, Bowman EP, Yuan W, et al. CCL27-CCR10 interactions regulate T cell-mediated skin inflammation. Nature medicine 2002;8(2):157–65. [DOI] [PubMed] [Google Scholar]
- Hopfner KP, Hornung V. Molecular mechanisms and cellular functions of cGAS-STING signalling. Nat Rev Mol Cell Biol 2020;21(9):501–21. [DOI] [PubMed] [Google Scholar]
- Hussein MR, Aboulhagag NM, Atta HS, Atta SM. Evaluation of the profile of the immune cell infiltrate in lichen planus, discoid lupus erythematosus, and chronic dermatitis. Pathology 2008;40(7):682–93. [DOI] [PubMed] [Google Scholar]
- Ioannides D, Golden BD, Buyon JP, Bystryn JC. Expression of SS-A/Ro and SS-B/La antigens in skin biopsy specimens of patients with photosensitive forms of lupus erythematosus. Archives of dermatology 2000;136(3):340–6. [DOI] [PubMed] [Google Scholar]
- Jones SK. Ultraviolet radiation (UVR) induces cell-surface Ro/SSA antigen expression by human keratinocytes in vitro: a possible mechanism for the UVR induction of cutaneous lupus lesions. The British journal of dermatology 1992;126(6):546–53. [DOI] [PubMed] [Google Scholar]
- Katayama S, Panelius J, Koskenmies S, Skoog T, Mähönen K, Kisand K, et al. Delineating the Healthy Human Skin UV Response and Early Induction of Interferon Pathway in Cutaneous Lupus Erythematosus. J Invest Dermatol 2019;139(9):2058–61.e4. [DOI] [PubMed] [Google Scholar]
- Kemp MG, Lindsey-Boltz LA, Sancar A. UV Light Potentiates STING (Stimulator of Interferon Genes)-dependent Innate Immune Signaling through Deregulation of ULK1 (Unc51-like Kinase 1). J Biol Chem 2015;290(19):12184–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köck A, Schwarz T, Kirnbauer R, Urbanski A, Perry P, Ansel JC, et al. Human keratinocytes are a source for tumor necrosis factor alpha: evidence for synthesis and release upon stimulation with endotoxin or ultraviolet light. The Journal of experimental medicine 1990;172(6):1609–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kripke ML, Fisher MS. Immunologic parameters of ultraviolet carcinogenesis. J Natl Cancer Inst 1976;57(1):211–5. [DOI] [PubMed] [Google Scholar]
- Kuhn A, Herrmann M, Kleber S, Beckmann-Welle M, Fehsel K, Martin-Villalba A, et al. Accumulation of apoptotic cells in the epidermis of patients with cutaneous lupus erythematosus after ultraviolet irradiation. Arthritis Rheum 2006;54(3):939–50. [DOI] [PubMed] [Google Scholar]
- Kuhn A, Landmann A. The classification and diagnosis of cutaneous lupus erythematosus. J Autoimmun 2014;48–49:14–9. [DOI] [PubMed] [Google Scholar]
- Lauffer F, Jargosch M, Krause L, Garzorz-Stark N, Franz R, Roenneberg S, et al. Type I Immune Response Induces Keratinocyte Necroptosis and Is Associated with Interface Dermatitis. The Journal of investigative dermatology 2018;138(8):1785–94. [DOI] [PubMed] [Google Scholar]
- Lawley W, Doherty A, Denniss S, Chauhan D, Pruijn G, van Venrooij WJ, et al. Rapid lupus autoantigen relocalization and reactive oxygen species accumulation following ultraviolet irradiation of human keratinocytes. Rheumatology (Oxford, England) 2000;39(3):253–61. [DOI] [PubMed] [Google Scholar]
- Li X, Shu C, Yi G, Chaton CT, Shelton CL, Diao J, et al. Cyclic GMP-AMP synthase is activated by double-stranded DNA-induced oligomerization. Immunity 2013;39(6):1019–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XD, Wu J, Gao D, Wang H, Sun L, Chen ZJ. Pivotal roles of cGAS-cGAMP signaling in antiviral defense and immune adjuvant effects. Science 2013;341(6152):1390–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Berthier CC, Kahlenberg JM. Enhanced Inflammasome Activity in Systemic Lupus Erythematosus Is Mediated via Type I Interferon-Induced Up-Regulation of Interferon Regulatory Factor 1. Arthritis & rheumatology (Hoboken, NJ) 2017;69(9):1840–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie KJ, Carroll P, Martin CA, Murina O, Fluteau A, Simpson DJ, et al. cGAS surveillance of micronuclei links genome instability to innate immunity. Nature 2017;548(7668):461–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLeod AS, Rudolph R, Corriden R, Ye I, Garijo O, Havran WL. Skin-resident T cells sense ultraviolet radiation-induced injury and contribute to DNA repair. J Immunol 2014;192(12):5695–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamyrova G, Rider LG, Ehrlich A, Jones O, Pachman LM, Nickeson R, et al. Environmental factors associated with disease flare in juvenile and adult dermatomyositis. Rheumatology (Oxford) 2017;56(8):1342–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh N, James I, Maddison P. Clinical significance of antibodies to a 68 kDa U1RNP polypeptide in connective tissue disease. The Journal of rheumatology 1990;17(10):1320–8. [PubMed] [Google Scholar]
- Means TK, Latz E, Hayashi F, Murali MR, Golenbock DT, Luster AD. Human lupus autoantibody-DNA complexes activate DCs through cooperation of CD32 and TLR9. The Journal of clinical investigation 2005;115(2):407–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meller S, Winterberg F, Gilliet M, Müller A, Lauceviciute I, Rieker J, et al. Ultraviolet radiation-induced injury, chemokines, and leukocyte recruitment: An amplification cycle triggering cutaneous lupus erythematosus. Arthritis and rheumatism 2005;52(5):1504–16. [DOI] [PubMed] [Google Scholar]
- Menéndez A, Gómez J, Caminal-Montero L, Díaz-López JB, Cabezas-Rodríguez I, Mozo L. Common and specific associations of anti-SSA/Ro60 and anti-Ro52/TRIM21 antibodies in systemic lupus erythematosus. TheScientificWorldJournal 2013;2013:832789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menke J, Hsu MY, Byrne KT, Lucas JA, Rabacal WA, Croker BP, et al. Sunlight triggers cutaneous lupus through a CSF-1-dependent mechanism in MRL-Fas(lpr) mice. J Immunol 2008;181(10):7367–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mond CB, Peterson MG, Rothfield NF. Correlation of anti-Ro antibody with photosensitivity rash in systemic lupus erythematosus patients. Arthritis and rheumatism 1989;32(2):202–4. [DOI] [PubMed] [Google Scholar]
- Morales J, Homey B, Vicari AP, Hudak S, Oldham E, Hedrick J, et al. CTACK, a skin-associated chemokine that preferentially attracts skin-homing memory T cells. Proceedings of the National Academy of Sciences of the United States of America 1999;96(25):14470–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motwani M, Pesiridis S, Fitzgerald KA. DNA sensing by the cGAS-STING pathway in health and disease. Nat Rev Genet 2019;20(11):657–74. [DOI] [PubMed] [Google Scholar]
- Neubert E, Bach KM, Busse J, Bogeski I, Schön MP, Kruss S, et al. Blue and Long-Wave Ultraviolet Light Induce in vitro Neutrophil Extracellular Trap (NET) Formation. Front Immunol 2019;10:2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien JC, Hosler GA, Chong BF. Changes in T cell and B cell composition in discoid lupus erythematosus skin at different stages. Journal of dermatological science 2017;85(3):247–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada S, Weatherhead E, Targoff IN, Wesley R, Miller FW. Global surface ultraviolet radiation intensity may modulate the clinical and immunologic expression of autoimmune muscle disease. Arthritis Rheum 2003;48(8):2285–93. [DOI] [PubMed] [Google Scholar]
- Pablos JL, Santiago B, Galindo M, Carreira PE, Ballestin C, Gomez-Reino JJ. Keratinocyte apoptosis and p53 expression in cutaneous lupus and dermatomyositis. J Pathol 1999;188(1):63–8. [DOI] [PubMed] [Google Scholar]
- Parks CG, Wilkerson J, Rose KM, Faiq A, Noroozi Farhadi P, Long CS, et al. Association of Ultraviolet Radiation Exposure With Dermatomyositis in a National Myositis Patient Registry. Arthritis Care Res (Hoboken) 2020;72(11):1636–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattison DI, Davies MJ. Actions of ultraviolet light on cellular structures. Exs 2006(96):131–57. [DOI] [PubMed] [Google Scholar]
- Pattison DI, Rahmanto AS, Davies MJ. Photo-oxidation of proteins. Photochemical & photobiological sciences : Official journal of the European Photochemistry Association and the European Society for Photobiology 2012;11(1):38–53. [DOI] [PubMed] [Google Scholar]
- Paz-Elizur T, Sevilya Z, Leitner-Dagan Y, Elinger D, Roisman LC, Livneh Z. DNA repair of oxidative DNA damage in human carcinogenesis: potential application for cancer risk assessment and prevention. Cancer letters 2008;266(1):60–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter Kind PL, Plewig G. Phototesting in Lupus Erythematosus. Journal of Investigative Dermatology 1993;100(1, Supplement):S53–S7. [DOI] [PubMed] [Google Scholar]
- Piskin G, Bos JD, Teunissen MB. Neutrophils infiltrating ultraviolet B-irradiated normal human skin display high IL-10 expression. Archives of dermatological research 2005;296(7):339–42. [DOI] [PubMed] [Google Scholar]
- Prantner D, Darville T, Nagarajan UM. Stimulator of IFN gene is critical for induction of IFN-beta during Chlamydia muridarum infection. J Immunol 2010;184(5):2551–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Psarras A, Alase A, Antanaviciute A, Carr IM, Md Yusof MY, Wittmann M, et al. Functionally impaired plasmacytoid dendritic cells and non-haematopoietic sources of type I interferon characterize human autoimmunity. Nature communications 2020;11(1):6149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rana S, Byrne SN, MacDonald LJ, Chan CY, Halliday GM. Ultraviolet B suppresses immunity by inhibiting effector and memory T cells. Am J Pathol 2008;172(4):993–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reefman E, Kuiper H, Limburg PC, Kallenberg CG, Bijl M. Type I interferons are involved in the development of ultraviolet B-induced inflammatory skin lesions in systemic lupus erythaematosus patients. Ann Rheum Dis 2008;67(1):11–8. [DOI] [PubMed] [Google Scholar]
- Ren Y, Tang J, Mok MY, Chan AW, Wu A, Lau CS. Increased apoptotic neutrophils and macrophages and impaired macrophage phagocytic clearance of apoptotic neutrophils in systemic lupus erythematosus. Arthritis and rheumatism 2003;48(10):2888–97. [DOI] [PubMed] [Google Scholar]
- Rongvaux A, Jackson R, Harman CC, Li T, West AP, de Zoete MR, et al. Apoptotic caspases prevent the induction of type I interferons by mitochondrial DNA. Cell 2014;159(7):1563–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabour Alaoui S, Dessirier V, de Araujo E, Alexaki VI, Pelekanou V, Lkhider M, et al. TWEAK affects keratinocyte G2/M growth arrest and induces apoptosis through the translocation of the AIF protein to the nucleus. PloS one 2012;7(3):e33609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders CJ, Van Weelden H, Kazzaz GA, Sigurdsson V, Toonstra J, Bruijnzeel-Koomen CA. Photosensitivity in patients with lupus erythematosus: a clinical and photobiological study of 100 patients using a prolonged phototest protocol. Br J Dermatol 2003;149(1):131–7. [DOI] [PubMed] [Google Scholar]
- Sarkar MK, Hile GA, Tsoi LC, Xing X, Liu J, Liang Y, et al. Photosensitivity and type I IFN responses in cutaneous lupus are driven by epidermal-derived interferon kappa. Annals of the rheumatic diseases 2018;77(11):1653–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer I, Schaljo B, Vogl C, Gattermeier I, Kolbe T, Müller M, et al. Interferons limit inflammatory responses by induction of tristetraprolin. Blood 2006;107(12):4790–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schornagel IJ, Sigurdsson V, Nijhuis EH, Bruijnzeel-Koomen CA, Knol EF. Decreased neutrophil skin infiltration after UVB exposure in patients with polymorphous light eruption. The Journal of investigative dermatology 2004;123(1):202–6. [DOI] [PubMed] [Google Scholar]
- Shi ZR, Cao CX, Tan GZ, Wang L. The association of serum anti-ribosomal P antibody with clinical and serological disorders in systemic lupus erythematosus: a systematic review and meta-analysis. Lupus 2015;24(6):588–96. [DOI] [PubMed] [Google Scholar]
- Shin MS, Kang Y, Lee N, Kim SH, Kang KS, Lazova R, et al. U1-small nuclear ribonucleoprotein activates the NLRP3 inflammasome in human monocytes. Journal of immunology (Baltimore, Md : 1950) 2012;188(10):4769–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin MS, Kang Y, Lee N, Wahl ER, Kim SH, Kang KS, et al. Self double-stranded (ds)DNA induces IL-1β production from human monocytes by activating NLRP3 inflammasome in the presence of anti-dsDNA antibodies. Journal of immunology (Baltimore, Md : 1950) 2013;190(4):1407–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipman WD, Chyou S, Ramanathan A, Izmirly PM, Sharma S, Pannellini T, et al. A protective Langerhans cell-keratinocyte axis that is dysfunctional in photosensitivity. Sci Transl Med 2018;10(454). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shreedhar VK, Pride MW, Sun Y, Kripke ML, Strickland FM. Origin and characteristics of ultraviolet-B radiation-induced suppressor T lymphocytes. J Immunol 1998;161(3):1327–35. [PubMed] [Google Scholar]
- Skopelja-Gardner S, An J, Tai J, Tanaka L, Sun X, Hermanson P, et al. The early local and systemic Type I interferon responses to ultraviolet B light exposure are cGAS dependent. Scientific reports 2020;10(1):7908-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skopelja-Gardner S, Tai J, Sun X, Tanaka L, Kuchenbecker JA, Snyder JM, et al. Acute skin exposure to ultraviolet light triggers neutrophil-mediated kidney inflammation. Proceedings of the National Academy of Sciences of the United States of America 2021;118(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solé C, Gimenez-Barcons M, Ferrer B, Ordi-Ros J, Cortés-Hernández J. Microarray study reveals a transforming growth factor-β-dependent mechanism of fibrosis in discoid lupus erythematosus. Br J Dermatol 2016;175(2):302–13. [DOI] [PubMed] [Google Scholar]
- Sontheimer C, Liggitt D, Elkon KB. Ultraviolet B Irradiation Causes Stimulator of Interferon Genes-Dependent Production of Protective Type I Interferon in Mouse Skin by Recruited Inflammatory Monocytes. Arthritis & rheumatology (Hoboken, NJ) 2017;69(4):826–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stannard JN, Reed TJ, Myers E, Lowe L, Sarkar MK, Xing X, et al. Lupus Skin Is Primed for IL-6 Inflammatory Responses through a Keratinocyte-Mediated Autocrine Type I Interferon Loop. The Journal of investigative dermatology 2017a;137(1):115–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stannard JN, Reed TJ, Myers E, Lowe L, Sarkar MK, Xing X, et al. Lupus Skin Is Primed for IL-6 Inflammatory Responses through a Keratinocyte-Mediated Autocrine Type I Interferon Loop. J Invest Dermatol 2017b;137(1):115–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturfelt G, Truedsson L, Johansen P, Jonsson H, Nived O, Sjöholm AG. Homozygous C4A deficiency in systemic lupus erythematosus: analysis of patients from a defined population. Clinical genetics 1990;38(6):427–33. [DOI] [PubMed] [Google Scholar]
- Takai E, Tsukimoto M, Harada H, Kojima S. Involvement of P2Y6 receptor in p38 MAPK-mediated COX-2 expression in response to UVB irradiation of human keratinocytes. Radiation research 2011;175(3):358–66. [DOI] [PubMed] [Google Scholar]
- Takashima A, Bergstresser PR. Impact of UVB radiation on the epidermal cytokine network. Photochemistry and photobiology 1996;63(4):397–400. [DOI] [PubMed] [Google Scholar]
- Takeuchi H, Gomi T, Shishido M, Watanabe H, Suenobu N. Neutrophil elastase contributes to extracellular matrix damage induced by chronic low-dose UV irradiation in a hairless mouse photoaging model. Journal of dermatological science 2010;60(3):151–8. [DOI] [PubMed] [Google Scholar]
- Tsoi LC, Gharaee-Kermani M, Berthier CC, Nault T, Hile GA, Estadt SN, et al. IL18-containing 5-gene signature distinguishes histologically identical dermatomyositis and lupus erythematosus skin lesions. JCI insight 2020;5(16). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsoi LC, Hile GA, Berthier CC, Sarkar MK, Reed TJ, Liu J, et al. Hypersensitive IFN Responses in Lupus Keratinocytes Reveal Key Mechanistic Determinants in Cutaneous Lupus. J Immunol 2019;202(7):2121–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukazaki N, Watanabe M, Shimizu K, Hamasaki Y, Katayama I. Photoprovocation test and immunohistochemical analysis of inducible nitric oxide synthase expression in patients with Sjögren’s syndrome associated with photosensitivity. Br J Dermatol 2002;147(6):1102–8. [DOI] [PubMed] [Google Scholar]
- Tumurkhuu G, Chen S, Montano EN, Ercan Laguna D, De Los Santos G, Yu JM, et al. Oxidative DNA Damage Accelerates Skin Inflammation in Pristane-Induced Lupus Model. Frontiers in immunology 2020;11:554725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnier JL, Pachman LM, Lowe L, Tsoi LC, Elhaj S, Menon R, et al. Comparison of lesional juvenile myositis and lupus skin reveals overlapping yet unique disease pathophysiology. Arthritis Rheumatol 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Nguyen H, Di Girolamo N, Jackson N, Hampartzoumian T, Bullpitt P, Tedla N, et al. Ultraviolet radiation-induced cytokines promote mast cell accumulation and matrix metalloproteinase production: potential role in cutaneous lupus erythematosus. Scandinavian journal of rheumatology 2011;40(3):197–204. [DOI] [PubMed] [Google Scholar]
- Vermi W, Riboldi E, Wittamer V, Gentili F, Luini W, Marrelli S, et al. Role of ChemR23 in directing the migration of myeloid and plasmacytoid dendritic cells to lymphoid organs and inflamed skin. The Journal of experimental medicine 2005;201(4):509–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villanueva E, Yalavarthi S, Berthier CC, Hodgin JB, Khandpur R, Lin AM, et al. Netting neutrophils induce endothelial damage, infiltrate tissues, and expose immunostimulatory molecules in systemic lupus erythematosus. J Immunol 2011;187(1):538–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Dong X, Yuan Z, Zuo Y, Wang J. SSA/Ro antigen expressed on membrane of UVB-induced apoptotic keratinocytes is pathogenic but not detectable in supernatant of cell culture. Chinese medical journal 1999;112(6):512–5. [PubMed] [Google Scholar]
- Watson RO, Bell SL, MacDuff DA, Kimmey JM, Diner EJ, Olivas J, et al. The Cytosolic Sensor cGAS Detects Mycobacterium tuberculosis DNA to Induce Type I Interferons and Activate Autophagy. Cell Host Microbe 2015;17(6):811–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White MJ, McArthur K, Metcalf D, Lane RM, Cambier JC, Herold MJ, et al. Apoptotic caspases suppress mtDNA-induced STING-mediated type I IFN production. Cell 2014;159(7):1549–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf SJ, Estadt SN, Theros J, Moore T, Ellis J, Liu J, et al. Ultraviolet light induces increased T cell activation in lupus-prone mice via type I IFN-dependent inhibition of T regulatory cells. J Autoimmun 2019;103:102291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong D, Kea B, Pesich R, Higgs BW, Zhu W, Brown P, et al. Interferon and biologic signatures in dermatomyositis skin: specificity and heterogeneity across diseases. PLoS One 2012;7(1):e29161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wouters CH, Diegenant C, Ceuppens JL, Degreef H, Stevens EA. The circulating lymphocyte profiles in patients with discoid lupus erythematosus and systemic lupus erythematosus suggest a pathogenetic relationship. The British journal of dermatology 2004;150(4):693–700. [DOI] [PubMed] [Google Scholar]
- Xie Y, Jinnin M, Zhang X, Wakasugi S, Makino T, Inoue Y, et al. Immunohistochemical characterization of the cellular infiltrate in discoid lupus erythematosus. Bioscience trends 2011;5(2):83–8. [DOI] [PubMed] [Google Scholar]
- Yarosh D, Both D, Kibitel J, Anderson C, Elmets C, Brash D, et al. Regulation of TNFalpha production and release in human and mouse keratinocytes and mouse skin after UV-B irradiation. Photodermatology, photoimmunology & photomedicine 2000;16(6):263–70. [DOI] [PubMed] [Google Scholar]
- Yin Q, Xu X, Lin Y, Lv J, Zhao L, He R. Ultraviolet B irradiation induces skin accumulation of plasmacytoid dendritic cells: a possible role for chemerin. Autoimmunity 2014;47(3):185–92. [DOI] [PubMed] [Google Scholar]
- Zahn S, Graef M, Patsinakidis N, Landmann A, Surber C, Wenzel J, et al. Ultraviolet light protection by a sunscreen prevents interferon-driven skin inflammation in cutaneous lupus erythematosus. Exp Dermatol 2014;23(7):516–8. [DOI] [PubMed] [Google Scholar]