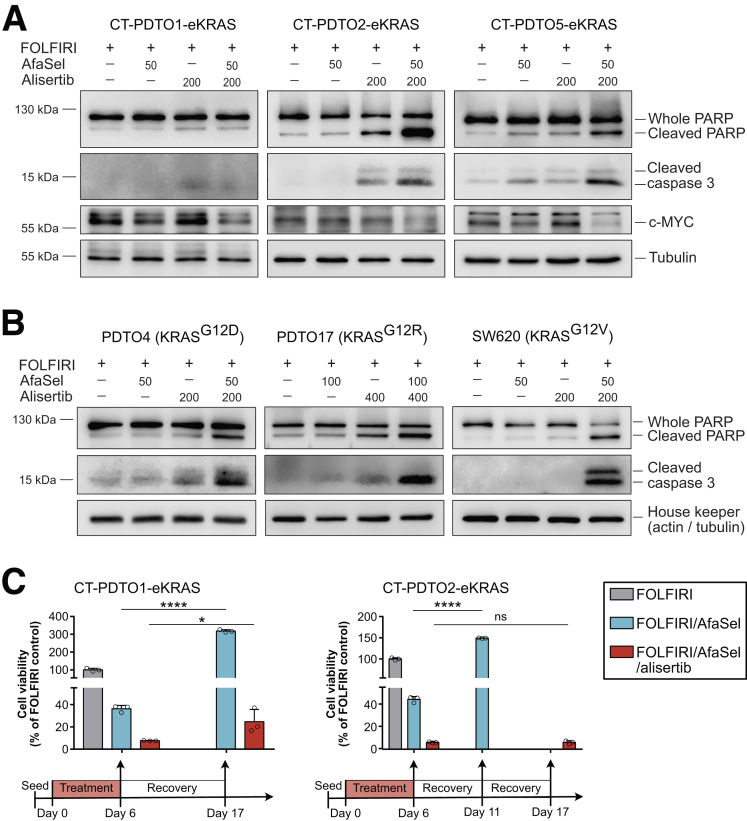
Figure 9.
AURKA inhibition in RAS mutant PDTOs primed by dual EGFR–MEK–ERK pathway blockade elicits apoptosis. (A and B) Immunoblot analysis of the indicated apoptosis markers and c-MYC in (A) CT–PDTOs carrying CRISPR/Cas9-engineered KRASG12D (eKRAS) and in (B) PDTOs and SW620 cells harboring the indicated oncogenic KRAS variants 2 days after treatment with the indicated agents (FOLFIRI, afatinib [dual EGFR/HER2 inhibitor], selumetinib [MEK inhibitor], alisertib [AURKA inhibitor]). Applied drug doses are given in nanomolar concentrations. (C) Cell viability assessment of CT–PDTOs that have been recovered (drug withdrawal) for up to 11 days after treatment with either FOLFIRI/AfaSel (blue bars) or FOLFIRI/AfaSel/alisertib for 6 days (red bars). All values are normalized to the FOLFIRI-treated control group on day 6 when drug removal had been performed (grey bars). Note that in case of CT–PDTO2–eKRAS (right panel), FOLFIRI/AfaSel-treated PDTOs were completely regrown on day 11 and therefore this measurement was performed at an earlier time point compared with the FOLFIRI/AfaSel/alisertib-treated CT–PDTO2–eKRAS (day 17). Statistical significance was assessed by 2-way ANOVA plus the Sidak multiple comparisons test and is indicated by asterisks (∗P ≤ .05, ∗∗∗∗P ≤ .0001, ns, non-significant: P > .9999. Means ± SD, n = 3.