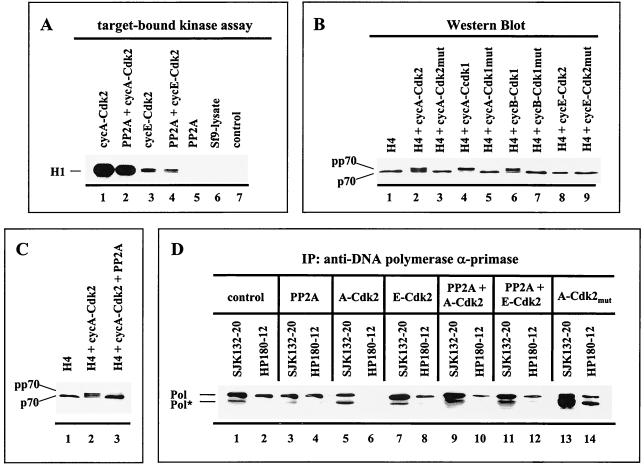
FIG. 2.
Cyclin A- and cyclin B-dependent kinases induce a p70 mobility shift that is reversible with PP2A and correlates with the abrogation of the immunoreactivity of the monoclonal anti-Pol α antibody HP180-12. (A) Fifty micrograms of lysates from baculovirus-infected cells was immunoprecipitated (IP) with the monoclonal antibody 12CA5 specific for the HA-tagged kinase and tested for kinase activity with 1 μg of H1 added as a substrate (lanes 1 to 4). 12CA5-immunoprecipitates, which expressed recombinant PP2A only (lane 5), and mock-infected insect cells (lane 6) were analyzed in an analogous target-bound kinase assay. The control reaction mixture consisted of kinase buffer and H1 (lane 7). Reactions were separated by SDS-PAGE (10% polyacrylamide) and visualized by autoradiography. (B) Five micrograms of lysates that express the four subunits of Pol-Prim (H4) in the absence (lane 1) or presence of the indicated recombinant Cdks (lanes 2 to 9) was subjected to SDS-PAGE (8% polyacrylamide). The p70 subunit was detected by Western blotting with an anti-p70 antiserum (1:5,000). (C) Recombinant Pol-Prim was coexpressed with the modifying enzymes as indicated. Five micrograms of lysates was separated by SDS-PAGE (8% polyacrylamide), and p70 was detected as described above. (D) Recombinant Pol-Prim (lanes 1 and 2) coexpressed with modifying enzymes, as indicated in lanes 3 to 14, was immunoprecipitated (IP) with anti-p180 monoclonal antibodies SJK132-20 and HP180-12. Immunoprecipitates obtained from equal amounts of protein from total-cell lysates were immunoblotted and analyzed with monoclonal anti-p180 antibody HP180-7 (1:5). Pol* indicates a 165-kDa degradation product of Pol α.