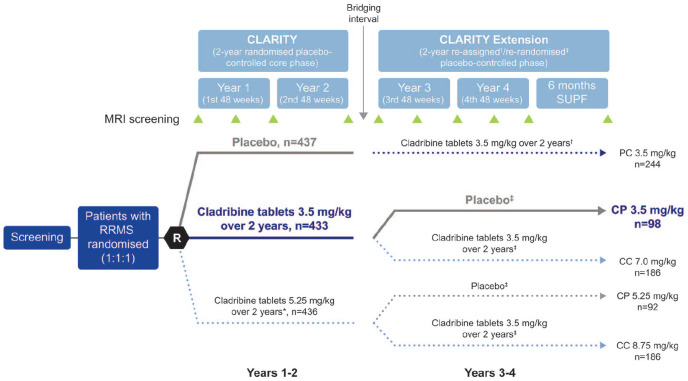
Figure 1.
CLARITY and CLARITY Extension study design.
Adapted from the study by Giovannoni et al. 13
*Cladribine tablets 3.5 mg/kg over 2 years is the only approved dose.
†Patients were re-assigned to cladribine tablets 3.5 mg/kg upon entry to CLARITY Extension.
‡Patients were re-randomised upon entry to CLARITY Extension.
CP 3.5 mg/kg: cladribine tablets 3.5 mg/kg in CLARITY followed by placebo in CLARITY Extension; CP 5.25 mg/kg: cladribine tablets 5.25 mg/kg in CLARITY followed by placebo in CLARITY Extension; CC 7 mg/kg: cladribine tablets 3.5 mg/kg in CLARITY followed by cladribine tablets 3.5 mg/kg in CLARITY Extension; CC 8.75 mg/kg: cladribine tablets 5.25 mg/kg in CLARITY followed by cladribine tablets 3.5 mg/kg in CLARITY Extension; PC 3.5 mg/kg: placebo in CLARITY followed by cladribine tablets 3.5 mg/kg in CLARITY Extension; SUPF: supplemental follow-up.
n: numbers are representative of the intention-to-treat population.
Treatment groups shown in coloured and bolded text were analysed in this study.