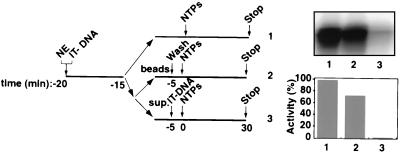
FIG. 2.
Functional Pol I transcription PICs captured from nuclear extracts (NEs) onto immobilized promoter templates. The experimental design to test for the isolation of PICs is outlined. Five microliters of immobilized template (50 ng of Fr4 per μl of beads [IT-DNA]) was incubated for 5 min at 4°C with 2 μl of HeLa NE in a 10-μl reaction volume and washed with TM10i–0.05 M KCl buffer. The reaction mixture was split equally into two. In one portion, the immobilized template was left in the NE for the entire time (lane 1), while in the other the beaded template was separated (lanes 2 and 3). The beads were washed in TM10i–0.05 M KCl buffer before initiation of transcription (lane 2), and the supernatant was tested for transcriptional activity by adding back the immobilized promoter template. Transcription in all three reactions was initiated with the addition of ribonucleoside triphosphates NTPs, and the reactions were allowed to proceed for 30 min at 30°C. Transcript synthesis was analyzed by S1 nuclease protection. The autoradiograph shows the transcript levels from in vitro transcription reactions supported by HeLa cell NE and immobilized DNA (lane 1), by isolated PICs on the immobilized DNA (lane 2), and by HeLa cell NE after PIC extraction (lane 3). Phosphorimager quantitation is presented in a bar graph, with the signal in lane 1 set at 100%.