Abstract
Binding levels and neutralization activity of anti–type 1 interferon autoantibodies peaked during acute coronavirus disease 2019 and markedly decreased thereafter. Most patients maintained some ability to neutralize type 1 interferon into convalescence despite lower levels of binding immunoglobulin G. Identifying these autoantibodies in healthy individuals before the development of critical viral disease may be challenging.
Keywords: COVID-19, IFN-α, IFN-ω, autoantibodies, convalescence
Anti-cytokine autoantibodies (autoAbs) can phenocopy mendelian defects in immune-signaling pathways to cause susceptibility to severe disease [1]. It was previously reported that neutralizing autoAbs against type 1 (T1) interferons (IFNs), primarily IFN-α2 and/or IFN-ω, were present in 101 of 987 critically ill patients with coronavirus disease 2019 (COVID-19) from whom plasma or serum samples were obtained during hospitalization for acute disease, but in none of 663 asymptomatic or mildly symptomatic patients [2]. During this initial screening, the anti-T1IFN autoAbs showed high fluorescence intensity (FI) in a multiplex binding assay; patient plasma samples diluted to 10% were able to neutralize ≥10 ng/mL of IFN-α2 and/or IFN-ω.
Before the COVID-19 pandemic, neutralizing anti-T1IFN autoAbs of similar potency had been identified in patients with autoimmune polyendocrine syndrome type 1 (APS-1), hypomorphic recombination-activating gene (RAG) mutations, thymoma, and other diseases [3–5]. Within our cohort of patients with acute COVID-19, no causal genetic defect or underlying comorbid conditions has yet been found to account for the anti-T1IFN autoAbs. In 2 critically ill patients with COVID-19 who had samples available from before infection, neutralizing anti-T1IFN autoAbs were already present [2]. However, it is currently not known whether the neutralizing anti-T1IFN autoAbs in most patients predate critical COVID-19 infection as they do in patients with APS-1, RAG-deficient patients, and patients with thymoma, nor is it known whether they persist after acute COVID-19 has resolved.
METHODS
Serum or plasma samples were collected longitudinally from 13 critically ill patients from Italy with acute COVID-19 and from 10 COVID-19–negative patients with either APS-1, RAG deficiency, or thymoma who had detectable autoAbs against IFN-α2 and/or IFN-ω. Twelve of the 13 patients with acute COVID-19 had been included as part of a larger cohort in which single autoAb measurements were reported during early hospitalization [2]. Patients with acute COVID-19 were followed up for an average of 147 days (range, 71–236 days) after hospital admission for COVID-19 and other patients were followed up for an average of 269 days (65-451 days) after their respective diagnoses. Clinical and/or demographic data were collected (Supplementary Tables 1 and 2). All subjects were recruited according to protocols approved by local institutional review boards of Comitato Etico Provinciale (NP 4000–Studio CORONAlab) and the National Institute of Allergy and Infectious Diseases. All protocols followed local ethics recommendations, and informed consent was obtained when required.
Patient serum or plasma samples diluted 1:100 in phosphate-buffered saline (PBS) were screened for binding levels of anti-T1IFN autoAbs, using a multiplex particle-based assay in which magnetic beads of differential fluorescence were covalently coupled to recombinant human IFN-α2, IFN-β, or IFN-ω at lysine residues. The FI of the bound phycoerythrin (PE)-conjugated secondary anti-human IgG Fc was previously shown to be proportional to IgG binding levels of anti-T1IFN autoAbs [6]. FI measurements of anti-T1IFN autoAbs were corrected for background FI using a well containing phosphate-buffered saline alone. A corrected FI value over the threshold of 3 standard deviations above the mean for the 1230 healthy controls (HCs) was defined as high (1268 FI for anti-IFN- α2, 1392 FI for anti-IFN-ω autoAbs), as determined in a previous study [2]. Alternatively, to determine Ig isotypes, secondary biotinylated anti-human immunoglobulin (Ig) G, IgM, IgA, or IgE Fc were used, and levels were revealed using phycoerythrin-conjugated streptavidin.
The neutralizing activity of anti–T1IFN autoAbs was determined by assessing STAT1 phosphorylation on Y710 (pSTAT1) in HC peripheral blood mononuclear cells after 15 minutes of stimulation with 10 ng/mL of IFN-α2 or IFN-ω in the presence of 10% HC or patient serum or plasma samples. The level of pSTAT1 was assessed in CD14+ monocytes and normalized to that of 10% HC plasma; 0%–20% pSTAT1 was classified as neutralizing, 20%–65% as partially neutralizing, and >65% as not neutralizing [2]. A protein G column was used to deplete IgG from the plasma, the IgG-depleted flow-through fraction was collected, and the pSTAT1 level was assessed and compared with that of total plasma [2].
To determine the median inhibitory concentration (IC50), patient plasma or serum samples collected at a given time point were serially diluted 2–10-fold into human serum AB to assess pSTAT1 in the presence of patient plasma at final concentrations ranging from 10% to 0.000625%. The IC50 was interpolated from the sigmoidal 4-parameter curve, where x = log (concentration). Data were analyzed using FlowJo software, version 10, and graphed using GraphPad Prism 8 software.
RESULTS
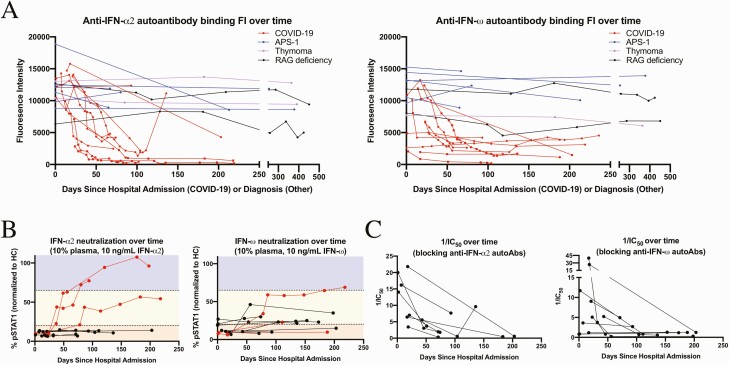
Consistent with previous observations of cross-reactivity against structurally and phylogenetically related T1IFN [2], 8 patients with COVID-19 in the current study had autoAbs against both IFN-α2 and IFN-ω, 2 against only IFN-α2, and 3 against only IFN-ω; none had autoAbs against IFN-β (Supplementary Tables 3 and 4). In the critically ill patients with COVID-19 and with anti-T1IFN autoAbs, binding IgG FI peaked during acute infection and markedly decreased thereafter (Figure 1A). The highest binding IgG FI was generally observed on the first available sample, obtained 0–24 days after hospital admission. Binding IgG FI predominated over IgM, while IgA or IgE FI were minimal (Supplementary Figures 1–14). In general, the anti-T1IFN binding IgG FIs in the acute COVID-19 cohort halved every approximately 2 weeks. This contrasted with the stable high FI anti-T1IFN IgG observed in patients with APS-1 or thymoma regardless of B-cell–depleting therapy with rituximab and in RAG-deficient patients despite hematopoietic stem cell transplantation (Figure 1A and Supplementary Tables 3 and 4).
Figure 1.
A, Anti–interferon (IFN) α2 and ω autoantibody (autoAb) binding fluorescence intensity (FI) over time, measured in days since hospital admission for patients with acute coronavirus disease 2019 (COVID-19) (red) and days since diagnosis for patients with autoimmune polyendocrine syndrome type 1 (APS-1) (blue), thymoma (purple), or recombination-activating gene protein (RAG) deficiency (black). B, Neutralizing activity over time in patients with COVID-19 over time against 10 ng/mL of IFN-α2 and/or IFN-ω at a plasma concentration of 10%. Black lines on the graph indicate that binding FI against the respective cytokine remained high throughout the course of follow-up; red lines, that binding FI against the respective cytokine dropped below threshold during follow-up. The STAT1 phosphorylation (pSTAT1) index was normalized against that of 10% health control (HC) plasma in the same experiment. Neutralizing (red region of graph) was defined as 0%–20% pSTAT1, partial neutralizing (yellow region) as 20%–65% pSTAT1, and not neutralizing (blue region) as >65% pSTAT1. C, Inverse median inhibitory concentration (1/IC50) over time for patients with COVID-19 in whom signaling to 10 ng/mL of IFN- α2 or IFN-ω was blocked with 10% plasma at all time points.
The neutralizing activity of anti-T1IFN autoAbs in patients with acute COVID-19, normalized to HCs, also tended to decrease over time, tracking with the drop in detectable IgG binding (Supplementary Figures 2–14). Three of the 10 patients who showed high binding FI of anti-IFN-α2 autoAbs during acute illness dropped before threshold in convalescence. Plasma diluted to 10% from 2 of these patients lost the ability to neutralize 10 ng/mL of IFN-α2 by approximately 40 and 54 days into our follow-up. Plasma from the third patient maintained the ability to partially neutralize 10 ng/mL of IFN-α2 up to 218 days after hospital admission, despite having subthreshold binding FI. Three of the 11 patients with high binding FI of anti-IFN-ω autoAbs during acute illness dropped to subthreshold, and 2 of those 3 maintained the ability to neutralize 10 ng/mL of IFN-ω at 10% plasma (Figure 1B and Supplementary Tables 3 and 4). In all 3 patients who lost detectable IgG FI but maintained neutralizing activity, in vitro preincubations to deplete IgG removed the neutralizing activity completely (Supplementary Figure 15).
To further characterize changes in neutralizing strength of anti-T1IFN autoAbs in patients with COVID-19 during disease and/or convalescence, IC50 values were determined against 10 ng/mL of the respective cytokine. For most patients, the neutralizing strength of the autoAbs decreased sharply after acute infection (Figure 1C and Supplementary Tables 3 and 4).
DISCUSSION
By following up patients from acute disease through convalescence, we showed that the anti-T1IFN autoAb responses in most of the tested patients with COVID-19 were highly dynamic, in sharp contrast to the stable high FI anti-T1IFN IgG in patients with APS-1 or thymoma and in RAG-deficient patients. Interestingly, the patients with COVID-19 with only anti-IFN-ω autoAbs showed a different pattern, with binding FI and/or neutralizing activity that remained constant or slightly increased over time (Supplementary Table 4), suggesting different inductive conditions.
Most patients with COVID-19 maintained some ability to neutralize T1IFN into convalescence. In several patients, this neutralizing activity persisted even after binding FI dropped to below detectable levels. We suspect that their neutralizing autoAbs recognized an epitope on IFN that was blocked by coupling the IFN to beads at lysine residues for our multiplex particle-based assay. Alternative explanations, such as binding IgA FI or autoAbs against the common T1IFN receptor, interferon-α/β receptor (IFNAR) 1/2, seem unlikely, given that neutralizing activity not only was depleted by in vitro preincubation to deplete IgG specifically (Supplementary Figure 15) but also did not extend against all type I IFN tested, including IFN-β (data not shown).
Our results suggest that the increase in endogenous IFNs during early viral infection may trigger a memory response of high FI but transient autoAbs. Anti-IFN-α2 and anti-IFN-ω autoAbs in the same patient often followed different trajectories, suggesting a polyclonal response to different triggers (Supplementary Figures 2–14). Moreover, the persistent neutralizing activity despite undetectable binding IgG in several patients suggests that only a small fraction of the polyclonal autoAbs responsible for high binding FI during acute disease contribute to the neutralizing activity. Prospective studies enrolling patients with COVID-19 from the time of their first positive PCR result are needed to better define these potential mechanisms.
Our group’s previous report showed that individuals with anti-IFN-α2 and/or IFN-ω autoAbs constitute a group at significant risk for developing critical COVID-19 disease [2]. Identifying these individuals early in the course of disease for targeted intervention with compensatory therapeutics, such as inhaled IFN-β therapy, might alter their course of disease and improve outcomes [7]. However, our findings indicate that the dynamic nature of these autoAbs may make diagnosis difficult in healthy individuals before acute disease, at least if the level of autoAb FI sought for diagnostics is what we observed during acute disease. Neutralization activity may be a more appropriate diagnostic measure than binding FI, because some convalescent patients with subthreshold binding FI against T1IFN during convalescence continued to potently neutralize supraphysiological amounts of the respective IFN. Accordingly, a high-throughput method for detecting neutralizing activity against more physiological levels of IFN, which has been recently validated in patients with COVID-19, may be useful and appropriate [8].
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors thank Yu Zhang, Sarah Weber, Li Ding, Sandhya Xirasagar, Jason Barnett, and Helen Matthews for their assistance, and Polly Matzinger for helpful discussions.
Financial support. This work was supported by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health (grant numbers 1ZIAAI001265, 1ZIAAI001312, 1ZIAAI001270, 1ZIAAI001318), and by Regione Lombardia, Italy (project “Risposta immune in pazienti con COVID-19 e co-morbidità”).
Potential conflicts of interest. H. C. S. is a shareholder in Amgen, Eli Lilly, and Pfizer. All other authors report no potential conflicts.
The authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
Elana R Shaw, Laboratory of Clinical Immunology and Microbiology, National Institute of Allergy and Infectious Diseases, Bethesda, Maryland, USA.
Lindsey B Rosen, Laboratory of Clinical Immunology and Microbiology, National Institute of Allergy and Infectious Diseases, Bethesda, Maryland, USA.
Aristine Cheng, Laboratory of Clinical Immunology and Microbiology, National Institute of Allergy and Infectious Diseases, Bethesda, Maryland, USA; Infectious Diseases Division, Internal Medicine, National Taiwan University Hospital and National Taiwan University College of Medicine, Taipei, Taiwan.
Kerry Dobbs, Laboratory of Clinical Immunology and Microbiology, National Institute of Allergy and Infectious Diseases, Bethesda, Maryland, USA.
Ottavia M Delmonte, Laboratory of Clinical Immunology and Microbiology, National Institute of Allergy and Infectious Diseases, Bethesda, Maryland, USA.
Elise M N Ferré, Laboratory of Clinical Immunology and Microbiology, National Institute of Allergy and Infectious Diseases, Bethesda, Maryland, USA.
Monica M Schmitt, Laboratory of Clinical Immunology and Microbiology, National Institute of Allergy and Infectious Diseases, Bethesda, Maryland, USA.
Luisa Imberti, CREA Laboratory, Diagnostic Department, ASST Spedali Civili di Brescia, Brescia, Italy.
Virginia Quaresima, CREA Laboratory, Diagnostic Department, ASST Spedali Civili di Brescia, Brescia, Italy.
Michail S Lionakis, Laboratory of Clinical Immunology and Microbiology, National Institute of Allergy and Infectious Diseases, Bethesda, Maryland, USA.
Luigi D Notarangelo, Laboratory of Clinical Immunology and Microbiology, National Institute of Allergy and Infectious Diseases, Bethesda, Maryland, USA.
Steven M Holland, Laboratory of Clinical Immunology and Microbiology, National Institute of Allergy and Infectious Diseases, Bethesda, Maryland, USA.
Helen C Su, Laboratory of Clinical Immunology and Microbiology, National Institute of Allergy and Infectious Diseases, Bethesda, Maryland, USA.
References
- 1. Browne SK. Anticytokine autoantibody-associated immunodeficiency. Annu Rev Immunol 2014; 32:635–57. [DOI] [PubMed] [Google Scholar]
- 2. Bastard P, Rosen LB, Zhang Q, et al. . Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science 2020; 370:eabd4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Walter JE, Rosen LB, Csomos K, et al. . Broad-spectrum antibodies against self-antigens and cytokines in RAG deficiency. J Clin Invest 2015; 125:4135–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Meager A, Visvalingam K, Peterson P, et al. . Anti-interferon autoantibodies in autoimmune polyendocrinopathy syndrome type 1. PLoS Med 2006; 3:e289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Burbelo PD, Browne SK, Sampaio EP, et al. . Anti-cytokine autoantibodies are associated with opportunistic infection in patients with thymic neoplasia. Blood 2010; 116:4848–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ding L, Mo A, Jutivorakool K, Pancholi M, Holland SM, Browne SK.. Determination of human anticytokine autoantibody profiles using a particle-based approach. J Clin Immunol 2012; 32:238–45. [DOI] [PubMed] [Google Scholar]
- 7. Monk PD, Marsden RJ, Tear VJ, et al. . Safety and efficacy of inhaled nebulised interferon beta-1a (SNG001) for treatment of SARS-CoV-2 infection: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Respir Med 2021; 9:196–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bastard P, Gervais A, Voyer TL, et al. . Autoantibodies neutralizing type I IFNs are present in ~4% of uninfected individuals over 70 years old and account for ~20% of COVID-19 deaths. Sci Immunol 2021; 6:eabl4340. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.