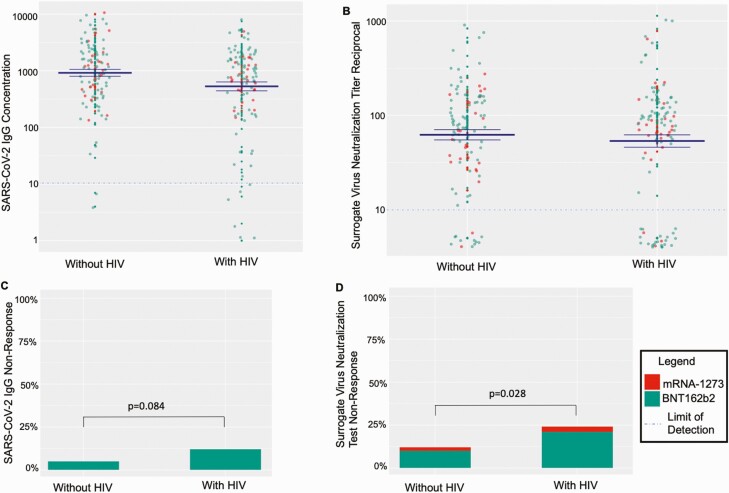
Figure 1.
Post-mRNA vaccination SARS-CoV-2 IgG concentrations and surrogate virus neutralization test reciprocal titers by HIV status and vaccine administered. The 4 panels demonstrate serologic responses to mRNA-based SARS-CoV-2 vaccination by HIV status. For all panels, green coloring indicates that the participant received the BNT162b2 vaccine, whereas red coloring indicates they received the mRNA-1273 vaccine. For panels A and B, the dotted lines show the assays’ lower limits of detection, and the solid blue lines indicate the mean ± 1 standard error. Panel A demonstrates SARS-CoV-2 IgG anti-receptor binding domain (RBD) concentrations in relative fluorescent units by HIV status. Panel B shows the SARS-CoV-2 surrogate virus neutralization antibody titer reciprocals by HIV status. Panel C demonstrates lack of SARS-CoV-2 anti-RBD IgG response as defined by response less than the assay lower limit of detection, <10 relative fluorescent units. Panel D demonstrates SARS-CoV-2 surrogate virus neutralization non-response as defined by below the assay lower limit of detection, <10 reciprocal titer. Abbreviations: HIV, human immunodeficiency virus; IgG, immunoglobulin G; mRNA, messenger RNA; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.