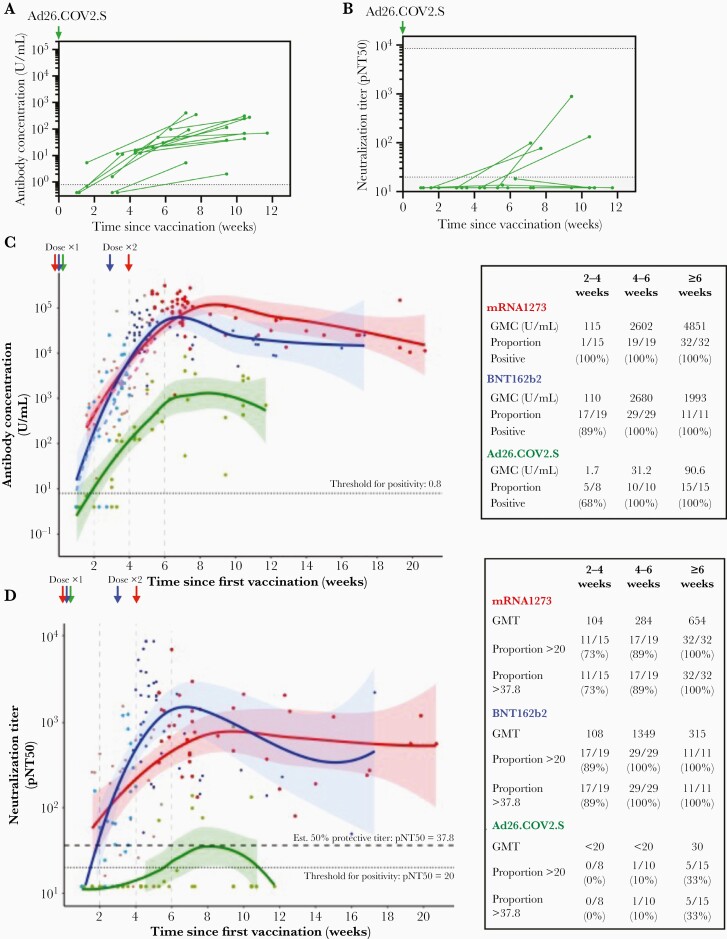
Figure 3.
Follow-up measurement of response in Ad26.COV2.S recipients and kinetics of humoral responses to mRNA1273, BNT162b2, and Ad26.COV2.S SARS-CoV-2 vaccines. Longitudinal assessment of SARS-CoV-2 spike IgG/A/M antibody titers (A) and virus neutralization (B) in 15 Ad26CoV2.S vaccinees with baseline and repeat measures. Pooling all data, we modelled the kinetics of antibody concentration and neutralization according to vaccine. C, SARS-CoV-2 spike IgG/A/M antibody levels and (D) virus neutralization for all donors sampled after vaccination, with best-fit lines (Loess fit) over the first 20 weeks following vaccination. C and D, GMC or titer, and proportion positive at threshold indicated are shown for the periods 0–2, 2–4, 4–6, and ≥6 weeks in the table inserts. Each individual point and corresponding fit are colored according to vaccine type (red, mRNA-1273; blue, BNT162b2; and green, Ad26COV2.S); shaded areas denote 95% confidence intervals, and points are additionally shaded according to dose. C, Dashed lines show best-fit lines for the period after receipt of the first dose of mRNA-1273 or BNT162b2. D, Dotted line denotes a pNT50 threshold of 20 derived from study of prepandemic controls, and the upper dashed line denotes a pNT50 titer of 37.8, which represents 20% of the geometric mean neutralization titers of unvaccinated individuals with prior infection and corresponds with 50% estimated protection in Khoury et al [4]. Abbreviations: GMC, geometric mean concentration; IgG/A/M, immunoglobulin G/A/M; pNT50, 50% pseudovirus neutralization titer; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.