Abstract
OBJECTIVE:
To examine the relationship between prenatal diagnostics (ultrasound examination and amniotic fluid Zika virus testing) and postnatal congenital Zika syndrome abnormalities.
DATA SOURCES:
Systematic searches were performed in 27 databases, including ClinicalTrials.gov, from inception to July 1, 2019, for articles with the keywords “Zika,” “prenatal,” “ultrasound,” and “amniocentesis.”
METHODS OF STUDY SELECTION:
A total of 3,049 unique records were identified. Two reviewers independently assessed titles, abstracts, and full texts for relevance; 84 articles met the inclusion criteria. These articles describe 402 mother-fetus or mother-neonate dyads; 385 were included in the review of prenatal ultrasound examination, and 56 in the review of amniocentesis (39 in both).
TABULATION, INTEGRATION, AND RESULTS:
Among 195 fetuses with congenital Zika syndrome findings on prenatal ultrasound examination, postnatal congenital Zika syndrome abnormalities were reported for 153 (78%; 95% CI 7–84%). High proportions of microcephaly (76%; 95% CI 69–82%) and brain abnormalities (78%; 95% CI 69–86%) were confirmed postnatally. Among 190 fetuses without congenital Zika syndrome findings on prenatal ultrasound examination, 17% (95% CI 12–24%) had congenital Zika syndrome abnormalities identified postnatally. Structural congenital Zika syndrome abnormalities were identified postnatally in approximately equal proportions among dyads with and without Zika virus RNA detected in an amniotic fluid specimen (68% and 67%; 95% CI 52–82% and 95% CI 38–88%). In six pregnancies, Zika virus RNA was detected in amniotic fluid but not in a subsequent amniocentesis specimen.
CONCLUSION:
Prenatal ultrasound examination frequently detects structural findings associated with Zika virus infection; however, not all abnormalities are detected, and some may represent transient findings. As with other congenital infections, prenatal detection may vary with timing of infection, timing of ultrasound examination, technical expertise, and severity of abnormalities. The detection of Zika virus RNA in amniotic fluid in the included studies did not predict the risk for congenital Zika syndrome abnormalities in these cases, and clearance of Zika virus RNA from amniotic fluid appears possible after maternal infection. Diagnostic testing for Zika virus infection remains a shared decision between patients and clinicians, and more data are needed to define clinical predictors that will inform these decisions.
SYSTEMATIC REVIEW REGISTRATION:
PROSPERO, CRD42018080959.
Zika virus infection during pregnancy poses a risk for adverse neonatal outcomes, including brain and eye abnormalities, and may result in a pattern of specific anomalies known as congenital Zika syndrome.1–4 As with other congenital infections, establishing a diagnosis of maternal Zika virus infection can guide detailed evaluation of fetal anatomy. The detection of abnormalities through serial ultrasonography facilitates planning for optimal clinical care of the pregnant mother and her child.
Fetal ultrasound findings associated with maternal Zika virus infection include intracranial calcifications, cortical atrophy and ventriculomegaly, abnormalities of cortical formation and of the corpus callosum, hypoplasia of the cerebellum and brainstem, microcephaly, and structural eye and limb abnormalities.5,6 Microcephaly has been specifically described as a late finding, first identified after 26 weeks of gestation.6 The Centers for Disease Control and Prevention (CDC) and the American College of Obstetricians and Gynecologists (ACOG) recommend serial prenatal ultrasound examinations for pregnant women with laboratory evidence of possible Zika virus infection to assess fetal anatomy, particularly neuroanatomy, and to monitor fetal growth.7,8 The sensitivity, specificity, positive and negative predictive values of prenatal ultrasound examination for congenital Zika syndrome remain unknown.
Amniocentesis is used in the diagnosis of several intrauterine infections (eg, cytomegalovirus, toxoplasmosis), and Zika virus RNA has been detected in amniotic fluid samples.9,10 The CDC and ACOG currently recommend that, if amniocentesis is indicated in the evaluation of abnormal prenatal findings, clinicians and families should consider Zika virus nucleic acid testing in the setting of possible maternal exposure to Zika virus.11 Routine amniocentesis is not recommended when the sole purpose is to detect Zika virus infection, because the sensitivity, specificity, and positive and negative predictive values of this testing for detection of fetal infection remain unknown.11
The CDC and ACOG recommendations are based on the assumption that prenatal diagnostics provide clinicians and families with timely information that can improve pregnancy outcomes. In this systematic review, we explore 1) the relationship between prenatal ultrasound findings and postnatal outcomes after congenital Zika virus exposure and 2) the relationship between amniotic fluid Zika virus test results and postnatal outcomes after congenital Zika virus exposure.
SOURCES
An expert research librarian searched the following 27 medical and public health databases for articles in the English language from inception to July 1, 2019: Academic Search Complete, CAB Abstracts, CI-NAHL, ClinicalTrials.gov, Cochrane Library, DART, Doctors without Borders, DTIC, EMBASE (OVID), Global Health (OVID), Google Scholar, JSTOR, LILACS, MEDLINE (OVID), NIH, NTIS (EbscoHost), Open Access Theses and Dissertations, PAHO, POPLINE, PsycINFO (OVID), PubMed, PMC (PubMed Central), Scopus, TOXLINE, UN Publications, WHO, and Worldcat.org. Example search terms and strategies are shown in Box 1. Duplicate articles were deleted from the literature search results using the automatic deduplicate function in Endnote software, and manually when additional duplicates were identified. We reviewed reference lists from articles identified by the database search, including key review articles, to identify additional primary references.
Box 1. PubMed Systematic Review Search Strategy.
#1
“Zika” OR “ZIKV” OR “Zika Virus” [MeSH] OR “Zika Virus Infection” [MeSH]
#2
“amniocentesis” or “amniotic” or “fetal blood” OR karyotype* or “Karyotype” [MeSH]) OR “Karyotyping” [MeSH] or “Amniocentesis” [MeSH] or “Fetal Blood” [MeSH] or “Amniotic Fluid” [MeSH]
#3
(“sonography” OR sonogram* or “MRI” or “magnetic resonance imaging” or “prenatal ultrasonography” or “Fetus/diagnostic imaging” [MeSH]) OR “echography” OR ultrasound* OR “imaging” [Title/Abstract])
AND
(“prenatal” OR “pre-natal” OR “Ultrasonography, Prenatal” [MeSH:noexp] or “fetus” or “fetuses” or “fetal” or “fetopathy”)
#4
(#1 and #2) OR (#1 and #3)
#5
Zika and cohort* and (neonat* or infant* or newborn)
STUDY SELECTION
We conducted title and abstract review of 3,039 unique records identified through the database search, and 10 articles identified during review of the references (Fig. 1).12,13 Two reviewers independently compared the full text of the 384 relevant articles with the inclusion criteria. Published articles were included if they contained case-level data regarding 1) congenital Zika virus exposure (Box 2), 2) the presence or absence of prenatal ultrasound findings consistent with possible congenital Zika syndrome (“prenatal congenital Zika syndrome findings,” Box 3) or amniotic fluid Zika virus test results, and 3) the presence or absence of structural neonatal or fetal outcomes potentially associated with Zika virus infection (see “congenital Zika syndrome abnormalities,” Box 3). “Postnatal outcomes” were identified based on clinical examination, imaging, laboratory testing, or pathology reports at birth, fetal loss, termination, or neonatal death. Cases with neural tube defects were excluded because evidence now indicates a lack of association of these defects with congenital Zika virus infection.14 Mother-fetus or mother-neonate dyads with a positive nucleic acid Zika virus test result were included, as well as those with an epidemiologic link to an area with risk of Zika virus infection during pregnancy and either positive congenital Zika syndrome postnatal outcomes or a positive serologic Zika virus test. In total, 84 articles were included: 77 case reports or case series and seven cohort studies. These articles describe 402 mother-fetus or mother-neonate dyads; 385 were included in the prenatal ultrasound analysis, and 56 were included in the amniocentesis analysis (39 were included in both) (Appendices 1 and 2, available online at http://links.lww.com/AOG/B820).
Fig. 1.
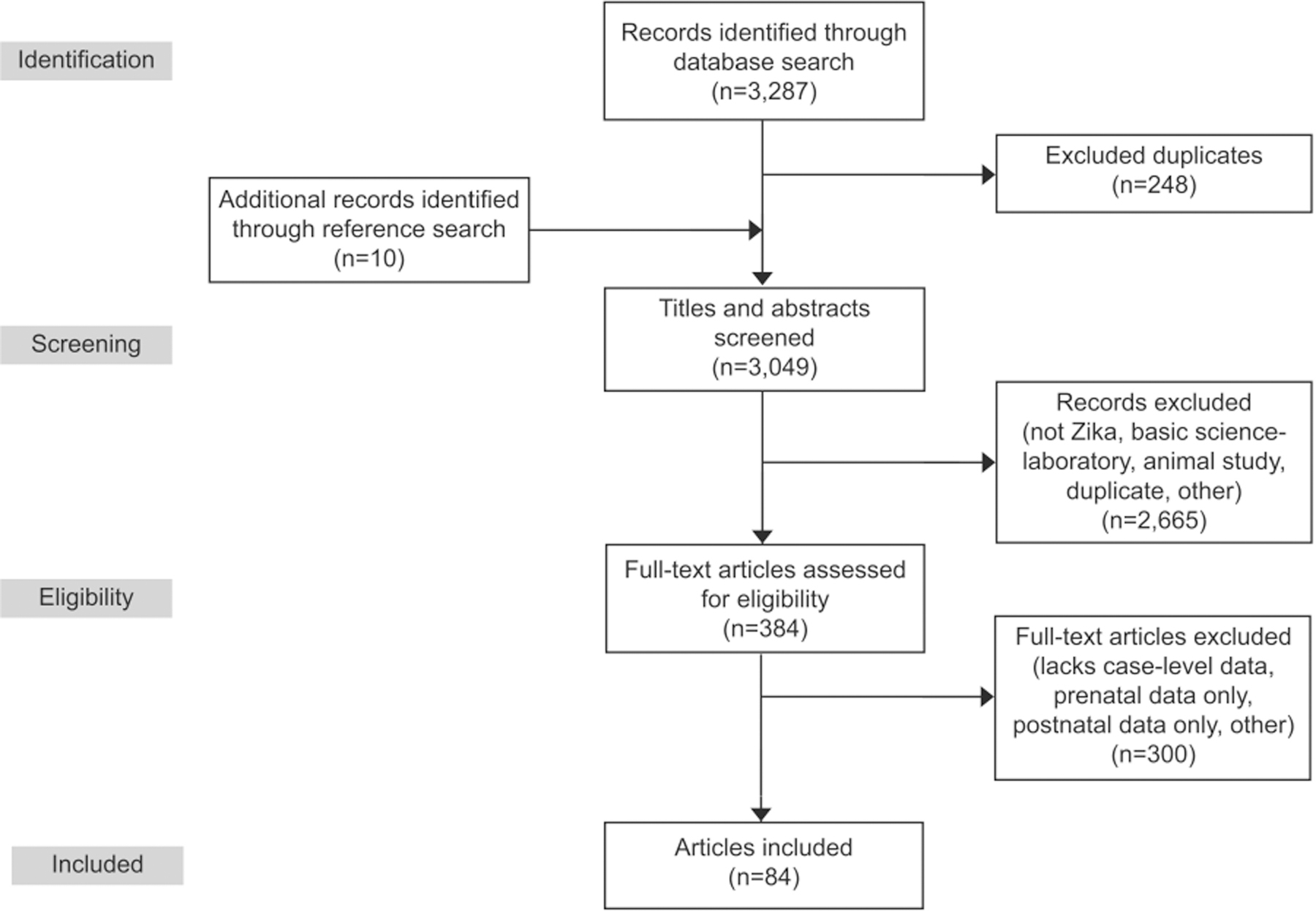
Prenatal diagnostics for congenital Zika syndrome systematic review PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flow diagram.
Box 2. Congenital Zika Virus Exposure.
Congenital Zika virus exposure was classified into three categories: confirmed, probable, and suspected.
Confirmed was defined by a positive nucleic acid test result for Zika virus infection on a maternal, fetal, or neonatal specimen (maternal serum, whole blood, or urine collected during gestation; amniotic fluid; placenta or cord blood; or neonatal serum, whole blood, urine, or cerebrospinal fluid (CSF) collected at or near birth).
Probable was defined by a positive serologic laboratory test result for Zika virus infection on a maternal, fetal, or neonatal specimen, and an epidemiologic link to an area with risk of Zika virus infection during gestation (maternal residence in, travel to, or sex without a condom with a partner with residence in or travel to such areas).
Suspected was defined by a structural congenital Zika syndrome abnormality (Box 3) in a neonate or fetus and an epidemiologic link to an area with risk of Zika virus infection during gestation.
Box 3. Prenatal Congenital Zika Syndrome Findings and Postnatal Congenital Zika Syndrome Abnormalities.
Prenatal congenital Zika syndrome findings include ultrasound findings consistent with possible congenital Zika virus infection:
Intracranial calcifications
Cortical atrophy and ventriculomegaly
Abnormalities of cortical formation and of the corpus callosum
Hypoplasia of the cerebellum and brainstem Microcephaly
Structural limb and eye abnormalities
Congenital Zika syndrome abnormalities include postnatal structural outcomes potentially associated with Zika virus infection:
Structural congenital Zika syndrome abnormalities include brain, limb, and eye abnormalities.
Brain abnormalities include microcephaly, intracranial calcifications; cerebral atrophy; abnormal cortical formation (eg, polymicrogyria, lissencephaly, pachygyria, schizencephaly, gray matter heterotopia); corpus callosum abnormalities; cerebellar abnormalities; and ventriculomegaly.
Limb abnormalities include arthrogryposis and talipes equinovarus.
Eye abnormalities include microphthalmia or anophthalmia; coloboma; cataracts; intraocular calcifications; chorioretinal anomalies involving the macula (eg, chorioretinal atrophy and scarring, macular pallor, gross pigmentary mottling); optic nerve atrophy, pallor, and other optic nerve abnormalities.
RESULTS
Data elements extracted from each manuscript included study design; details of maternal Zika virus exposure (Box 2); prenatal ultrasound examination timing, frequency, and findings; amniocentesis timing, frequency, and results; and neonatal or fetal outcomes. These data were compiled into an Excel spreadsheet; analysis was performed using SAS 9.4. Owing to heterogeneity across the included studies related to study population, study design, classification of exposure, prenatal diagnostic methods, and reported outcomes, analysis was limited to descriptive statistics.
Maternal Zika virus infections identified for inclusion in this review were predominantly symptomatic (n=323, 80%; 95% CI 76–84%). Most maternal infections were confirmed with nucleic acid testing (n=269, 67%; 95% CI 62–72%) and were reported in the first trimester (less than 14 weeks of gestation) (n=165 of the 305 with known timing information, 52%; 95% CI 46–58%). Among the 402 pregnancies included in this review, 354 (88%) resulted in live birth, 44 (11%; 95% CI 8–14%) in elective termination, and four (1%; 95% CI 0–3%) in pregnancy loss or stillbirth. Among the 354 liveborn neonates, Zika virus laboratory testing was reported on neonatal blood, urine, or cerebrospinal fluid samples from 134 (38%; 95% CI 33–43%); 14 neonates had positive nucleic acid test results, and 28 had positive serologic test results (Table 1).
Table 1.
Zika Virus Infection and Pregnancy Characteristics of the Mother-Fetus or Mother-Neonate Dyads Included in Systematic Reviews (N=402)
| Characteristic | Value |
|---|---|
| Symptomatic maternal Zika virus infection | 323 (80; 76–84) |
| Laboratory evidence of possible maternal Zika virus infection during pregnancy* | |
| NAT-confirmed Zika virus infection | 269 (67; 62–72) |
| IgM-positive only | 32 (8; 7–11) |
| IgM plus confirmatory PRNT-positive | 36 (9; 6–12) |
| Suspected Zika virus infection | 65 (16; 13–20) |
| Trimester of infection among mothers with symptoms or NAT-confirmed laboratory diagnosis in a maternal specimen† | |
| 1st | 1 65 (52; 46–58) |
| 2nd | 123 (39; 33–44) |
| 3rd | 29 (9; 6–13) |
| Pregnancy outcome | |
| Live birth | 329 (82; 78–85) |
| Gestational age (wk) | 38 (28–41) |
| Elective termination | 44 (11; 8–14) |
| Gestational age (wk) | 24 (17–39) |
| Pregnancy loss or stillbirth | 4 (1; 0–3) |
| Gestational age (wk) | 25 (12–32) |
| Neonatal death after live birth | 25 (6; 4–9) |
| Postnatal age (d) | 1 (1 d-2 mo)‡ |
| Neonatal Zika virus laboratory testing* | |
| Neonatal blood, urine, or CSF tested | 134/354 (38; 33–43) |
| Neonates with positive NAT result for Zika virus infection | 14/115 (12; 7–19) |
| Neonates with positive serology for Zika virus infection | 28/65 (43; 31–55) |
NAT, nucleic acid test; IgM, immunoglobulin M; PRNT, plaque reduction neutralization test; CSF, cerebrospinal fluid.
Data are n (%; 95% CI) or median (range).
Laboratory evidence categories were defined as follows: Confirmed = positive Zika virus NAT result on a maternal, fetal, or neonatal specimen (maternal serum, whole blood, or urine collected during gestation; amniotic fluid; placenta or cord blood; or neonatal serum, whole blood, urine, or CSF collected at or near birth). Probable=positive serologic test (IgM alone, IgM and confirmatory PRNT for Zika virus or flavivirus positive) on a maternal, fetal, or neonatal specimen (see Confirmed, above) with an epidemiologic link (residence in, travel to, or sex without a condom with a partner with residence or travel to an area with risk of Zika virus infection during gestation). Suspected=epidemiologic link (see Probable, above) with fetal or neonatal outcomes associated with congenital Zika syndrome (microcephaly or specific brain, limb, eye, hearing, and neurologic abnormalities).
Trimester information was available for only 305 of the 402 dyads. Percentages are based on denominator of 305.
Timing information available for only 9 of the 25 neonates with known death after live birth.
At least one prenatal ultrasound examination was reported during pregnancy for 385 dyads exposed to Zika virus (Fig. 2). Ultrasound examination identified prenatal congenital Zika syndrome findings in 195 (51%, 95% CI 46–56%) of these fetuses. Among those with prenatally-detected congenital Zika syndrome findings on ultrasound examination, one or more postnatal congenital Zika syndrome abnormality was confirmed in 153 (78%, 95% CI 72–84%). Among fetuses with ultrasound examinations that did not detect any prenatal congenital Zika syndrome findings (n=190), 33 (17%, 95% CI 12–24%) had congenital Zika syndrome abnormalities identified postnatally; most of these had microcephaly detected postnatally (Appendix 3, available online at http://links.lww.com/AOG/B820). Postnatal confirmation of specific congenital Zika syndrome abnormalities reported on prenatal ultrasound examination varied.
Fig. 2.
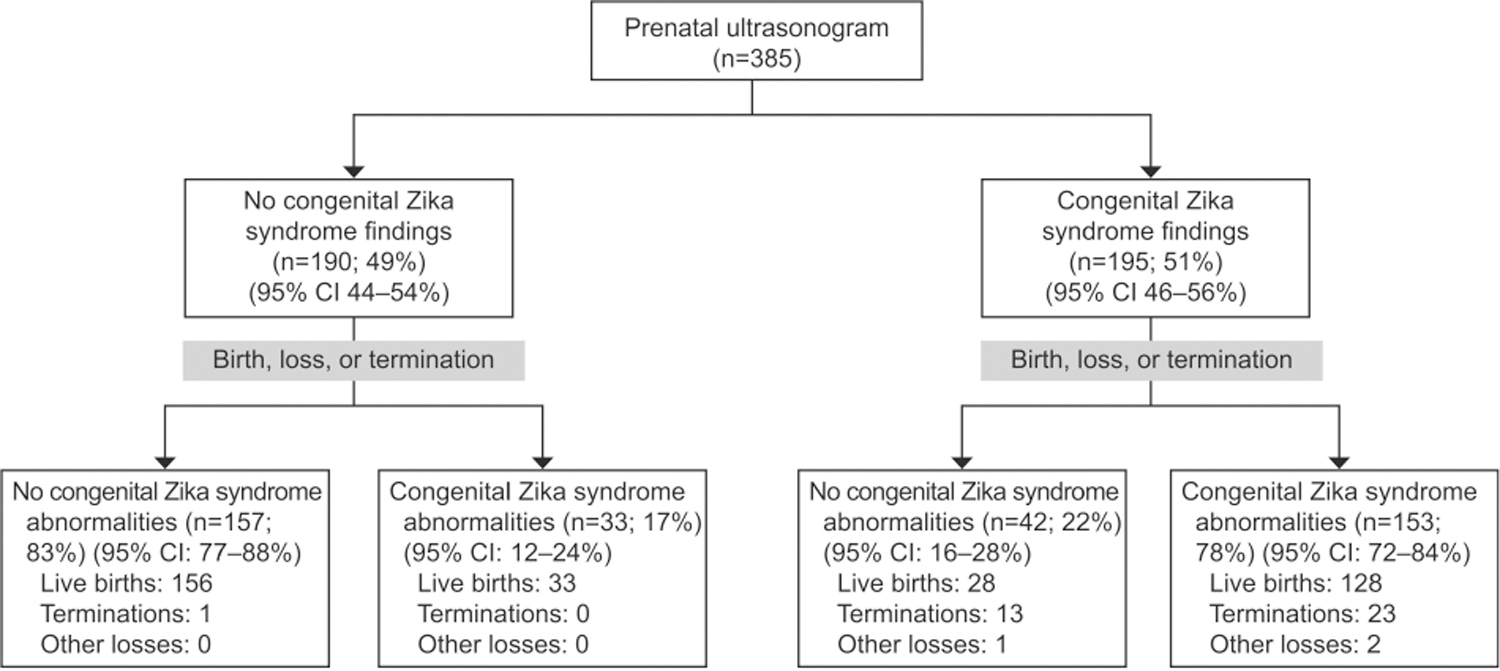
Congenital Zika syndrome outcomes after pregnancy completion among mother-fetus or mother-neonate dyads with one or more prenatal ultrasound examinations performed at any time during pregnancy.
Of fetuses with a prenatal ultrasound report of abnormal brain findings (n=143), 93 (65%, 95% CI 57–73%) had postnatal neuroimaging or autopsy. Among those with postnatal evaluation, 73 (78%, 95% CI 69–86%) had brain abnormalities confirmed (Table 2). Among pregnancies whose prenatal brain findings were not confirmed, 35 (50%, 95% CI 38–62%) had other postnatal congenital Zika syndrome abnormalities identified (including microcephaly, retinal atrophy, arthrogryposis, and talipes equinovarus). More than two-thirds of the cerebral atrophy (71%, 95% CI 48–89%), calcifications (68%,95% CI 54–80%), and ventriculomegaly (66%, 95% CI 53–77%) findings reported on prenatal ultrasound examination were subsequently confirmed postnatally.
Table 2.
Congenital Zika Syndrome Structural Abnormalities After Pregnancy Completion Among Those Reported on Prenatal Ultrasound Examination at Any Gestational Age
| Prenatal Ultrasound Examination |
||
|---|---|---|
| Prenatal Congenital Zika Syndrome Finding | Case Count* | Abnormality Confirmed by Postnatal Examination (Clinical Examination, Imaging, or Autopsy) |
| Microcephaly | 158 | 120 (76; 69–82) |
| Eye abnormalities | 5 | 2 (40; 5–85) |
| Limb abnormalities | 18 | 8 (44; 22–69) |
| Brain abnormalities | 143 | 73/93 (78; 69–86)† |
| Calcifications | 90 | 38/56 (68; 54–80) |
| Atrophy | 41 | 15/21 (71; 48–89)† |
| Abnormal cortical formation | 9 | 3/3 (100; 29–100)† |
| Corpus callosum abnormalities | 28 | 5/8 (63; 24–91)† |
| Cerebellar abnormalities | 38 | 8/20 (40; 19–64)† |
| Ventriculomegaly | 102 | 47/71 (66; 53–77)† |
Data are n or n (%; 95% CI).
Categories are not mutually exclusive; a fetus may appear in multiple rows.
Limited to those cases that reported any known neuroimaging or autopsy.
Prenatal microcephaly was identified in 158 and confirmed postnatally in 120 (76%, 95% CI 69–82%) dyads. Among the 38 neonates and fetuses without postnatal confirmation of a microcephaly finding, 11% (95% CI 3–23%) were found to have other congenital Zika syndrome abnormalities (including brain abnormalities: intracranial calcifications, cerebral atrophy, abnormal cortical formation, corpus callosum abnormalities, cerebellar abnormalities, ventriculomegaly; eye abnormalities: microphthalmia, cataract, or macular lesions; and limb abnormalities: arthrogryposis, talipes equinovarus).
Prenatal eye findings, specifically intraocular calcifications and microphthalmia, were detected in five fetuses; microphthalmia was confirmed in two of these after birth, and intraocular calcifications were not reported postnatally. Among the three neonates whose prenatal report of eye findings was not confirmed at birth, one had congenital Zika syndrome brain abnormalities (including cerebral atrophy, abnormal cortical formation, ventriculomegaly, intracranial calcifications, and cerebellar abnormalities).
The prenatal congenital Zika syndrome limb findings arthrogryposis or talipes equinovarus were confirmed postnatally in 44% (95% CI 22–69%) of dyads. Among those dyads with a prenatal limb finding that was not confirmed postnatally, other congenital Zika syndrome abnormalities were identified in 50% (including microcephaly, brain abnormalities: intracranial calcifications, cerebral atrophy, abnormal cortical formation, corpus callosum abnormalities, cerebellar abnormalities, and ventriculomegaly; talipes equinovarus; and eye abnormalities: hypoplastic optical nerve, macular atrophy).
Among the 186 neonates or fetuses with prenatal congenital Zika syndrome abnormalities confirmed postnatally, prenatal serial ultrasonography was reported during 29 pregnancies (ultrasound examinations: median 3, range 2–8); 12 had normal prenatal ultrasound examination results followed by subsequent detection of congenital Zika syndrome findings (Table 3). For most of these pregnancies, symptomatic maternal Zika virus infection in the first trimester was reported and normal ultrasound examination results were described 10 weeks or less after the maternal Zika virus infection. The time from onset of symptomatic maternal infection to first abnormal congenital Zika syndrome ultrasound finding ranged from 7 to 23 weeks. Of note, prenatal congenital Zika syndrome findings were not detectable by 18–20 weeks of gestation in half (n=6) of these pregnancies.
Table 3.
Clinical Details of 10 Mother-Fetus or Mother-Neonate Dyads With Serial Ultrasonography, With Normal Followed by Abnormal Ultrasound Reports, With Congenital Zika Syndrome Abnormalities
| SR_ID (Reference) | Maternal ZIKV Infection, Weeks of Gestation | Last Normal Ultrasound Examination |
First Abnormal Ultrasound Examination |
Prenatal Ultrasound Examination Congenital Zika Syndrome Findings, Includes Abnormalities From All Examinations | Pregnancy Outcome | Fetal or Neonatal ZIKV Laboratory Test Results | Neonatal or Fetal Congenital Zika Syndrome Imaging and Clinical or Autopsy Examination Abnormalities |
|---|---|---|---|---|---|---|---|
| [wk of Gestation (wk Elapsed from Maternal Symptoms)] | |||||||
| 9 Benjamin et al17 | Symptomatic at 10 wk; ZIKV NAT-positive maternal plasma at time of symptoms | 14 (4) | 19 (9) | Microcephaly, cerebellar abnormalities (hypoplasia); ventriculomegaly | Termination at 24 wk | ZIKV NAT-positive cord blood | Autopsy: hypoplasia of the corpus callosum, meningoencephalitis, calcifications, dilated ventricles; high sloping forehead, flat philtrum, nuchal and sub mental edema, microcephaly |
| 38 Brasil et al18 | Symptomatic at 8 wk; ZIKV NAT-positive in maternal blood, urine, or both UNK timing | 17 (9) | 20 (12) | Cerebellar atrophy (small transverse diameter) | Live birth at 39 wk | UNK | US and CT: periventricular lesions, global cerebral atrophy, macular lesions, congenital dislocation of hip |
| 107 Driggers et al19 | Symptomatic at 12 wk; ZIKV NAT-positive maternal serum 4 and 9 wk after symptoms | 17 (5) | 19 (7) | Cerebral atrophy (cerebral mantle appeared to be thin); corpus callosum abnormalities (agenesis); ventriculomegaly | Termination at 21 wk | ZIKV NAT-positive fetal tissues and placenta, membranes, and umbilical cord | Autopsy: normal anatomy, cortical atrophy, corpus callosum not well visualized, no overt microscopic abnormalities of the eyes |
| 126 Mlakar et al20 | Symptomatic at 13 wk; no maternal testing reported | 20 (7) | 29 (16) | Microcephaly, intracranial calcifications; cerebellar abnormalities; ventriculomegaly | Termination at 32 wk | ZIKV NAT-positive fetal brain tissue; ZIKV NAT-negative placenta and other fetal tissues | Autopsy: micrencephaly, widely open sylvian fissures, small cerebellum and brain stem, almost complete agyria and internal hydrocephalus of the lateral ventricles, numerous variable-sized calcifications in the cortex and subcortical white matter in the frontal, parietal, and occipital lobes |
| 127 Moron et al21 | Symptomatic at 13 wk; ZIKV NAT-negative maternal serum 16 wk after symptoms | 22 (9) | 29 (16) | Microcephaly, intracranial calcifications; cerebral atrophy; abnormal cortical formation (lissencephaly, pachygyria); ventriculomegaly | Live birth at 39 wk | ZIKV IgM negative serum, ZIKV IgG positive serum | CT: overlapping cranial bones and preserved cranial sutures, gross calcifications in cortical and subcortical structures with cerebral atrophy, ventriculomegaly and enlarged subarachnoid space; T2-weighted MRI: cranial-facial disproportion (microcephaly), cortical and subcortical atrophy, lissencephaly, pachygyria, increased subarachnoid space, nonhypertensive ventriculomegaly. Axial swan-weighted MRI: gross cortical and subcortical calcifications and signs of corpus callosum hypoplasia; clinical microcephaly |
| 191 Perez et al22 | Symptomatic at 8 wk; ZIKV NAT-positive maternal serum 9 wk after symptoms | 12 (4) | 19 (11) | Ventriculomegaly with hydrocephalus, arthrogryposis | Termination at 21 wk | ZIKV NAT-positive umbilical cord and brain tissue; ZIKV NAT-negative placenta | Autopsy: fetal hydrocephalus with dilation of both lateral ventricles, multiple calcifications at cortical level and brainstem; flexion contracture and deformity of joints of all four limbs, extreme flexion of hips and crossed femurs, under-developed muscles with replacement of muscle by adipose tissue |
| 248 Suy et al23 | Symptomatic at 9 wk; ZIKV NAT-positive maternal serum UNK timing | 15 (6) | 20 (11) | Intracranial calcifications (brain parenchyma); cerebral atrophy (severe); corpus callosum abnormalities (shortened); ventriculomegaly (bilateral, mild) | Live birth at 37 wk | ZIKV NAT-negative in CSF, urine, serum, placenta, membranes, and umbilical cord | US and MRI: intracranial calcifications, cerebral atrophy, thinned corpus callosum, microcephaly |
| 250 Vesnaver24 | Symptomatic at 13 wk; no maternal ZIKV testing | 20 (7) | 29 (16) | Microcephaly, intracranial calcifications; cerebellar abnormalities (small cerebellum); ventriculomegaly | Termination at 32 wk or more | ZIKV NAT-positive fetal brain tissue, ZIKV NAT-negative placenta, other organs | Autopsy: almost complete agyria with very few gyri in the occipital lobes resembling pachygyria, irregular white calcifications at the cortical-white matter border in the frontal and parietal lobes, lateral ventricles dilated; clinical microcephaly |
| 252 Werner et al25 | Symptomatic at 12 wk; no maternal ZIKV testing | 21 (9) | 32 (20) | Intracranial calcifications, microcephaly | Live birth at 38 wk | UNK | US: subcortical and periventricular calcifications with ventricular dilatation; clinical microcephaly; CTat 10 d: microcephaly, cortical atrophy, brain calcifications and small anterior fontanel with premature closure of metopic and coronal sutures; MRI at 1 mo: cephalic circumference 32.0 cm, pachygyria, corpus callosal dysgenesis, subcortical frontoparietal brain calcifications |
| 253 Werner et al26 | Symptomatic at 10 wk; ZIKV serology 23 wk after symptoms | 29 (19) | 33 (23) | Microcephaly, intracranial calcifications (periventricular); abnormal cortical formation (lissencephaly); ventriculomegaly | Live birth at 37 wk | ZIKV NAT-positive urine, blood; ZIKV NAT-negative CSF | US: thinning of the frontoparietal parenchyma, calcifications on the frontal brain surfaces and corpus callosum dysgenesis; persistent loud crying, seizures of upper and lower left limbs; CT at 30 d: borderline ventriculomegaly, corpus callosum dysgenesis, parenchymal atrophy, widespread multiple brain calcifications, small anterior fontanel with overlapping metopic and coronal sutures; MRI at 1 mo: HC measuring 30.0 cm, tapered frontoparietal parenchyma, pachygyria, underdeveloped sulci and gyri, extensive calcifications particularly in subcortical areas, and dysgenesis of the corpus callosum, multiple subcortical frontoparietal calcifications |
| 356 Sulleiro et al27 | Symptomatic at 9 wk; ZIKV NAT-positive maternal serum 3 wk after symptoms | 15 (6) | 19 (10) | Bilateral ventriculomegaly | Live birth at 37 wk | ZIKV NAT- and IgM-negative neonatal serum | US, CT, and MRI: microcephaly with a thinned corpus callosum and brain atrophy with parenchymal calcifications. Clinical: severe microcephaly, craniofacial disproportion, partially collapsed skull, prominent occiput, and excess nuchal skin. Neurologic examination: irritability, hyperexcitability, exacerbation of the primitive reflexes, inconsolable crying, and joint contractures. |
| 357 Valdespino-Vazquez et al28 | Symptomatic at 14 wk; ZIKV NAT-negative maternal serum 14 wk after symptoms; ZIKV NAT-positive fetal tissue at autopsy | 24 (10) | 28 (14) | Microcephaly, enlarged lateral ventricles, oligohydramnios, cortical calcifications | Live birth at 30 wk; neonatal death at 4 h | ZIKV NAT-positive fetal tissue | Autopsy: microcephaly, micrognathia and retrognathia, low-set ears, depressed nasal bridge, arthrogryposis; hypoplastic cerebral lobes and brain stem, lissencephaly, ventriculomegaly, cerebral calcifications. |
SR_ID, systematic review identification number; ZIKV, Zika virus; NAT, nucleic acid test; UNK, unknown, US, ultrasonography; CT, computed tomography; IgM, immunoglobulin M; IgG, immunoglobulin G; MRI, magnetic resonance imageing; CSF, cerebrospinal fluid;
Of 56 pregnancies with reported results for Zika virus nucleic acid testing of an amniotic fluid specimen, 41 (73%) had at least one positive Zika virus nucleic acid test result (Fig. 3). Among those with Zika virus RNA detected in amniotic fluid, 28 (68%, 95% CI 52–82%) had at least one postnatal congenital Zika syndrome abnormality, and 23 had a Zika virus-positive nucleic acid test result from a neonatal or fetal tissue specimen. Among 15 pregnancies with a negative Zika virus nucleic acid test result from amniotic fluid testing, 10 (67%, 95% CI 38–88%) had at least one postnatal congenital Zika syndrome abnormality and three had a Zika virus-positive nucleic acid test result (Appendix 4, available online at http://links.lww.com/AOG/B820).
Fig. 3.
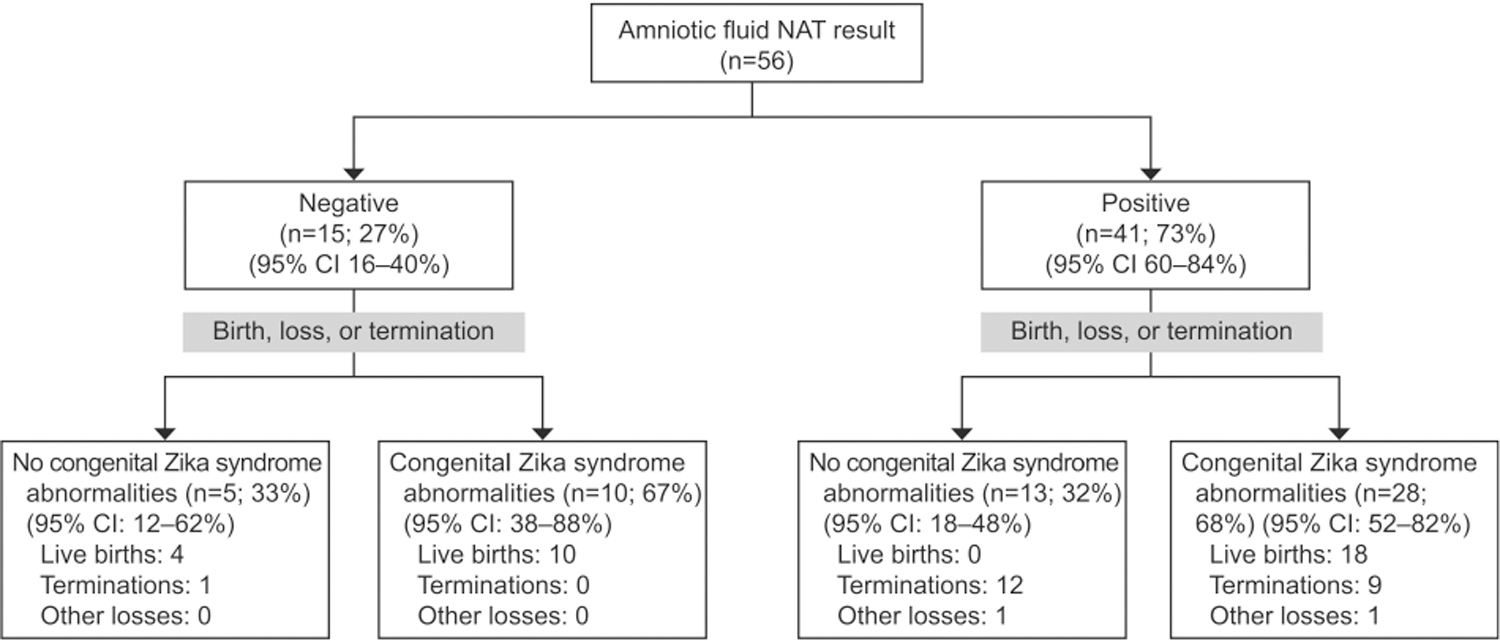
Congenital Zika syndrome outcomes after pregnancy completion among mother-fetus or mother-neonate dyads included in the systematic review with congenital Zika virus exposure and nucleic acid test (NAT) on an amniotic fluid specimen performed at any time during gestation.
Ten mothers had serial amniocenteses; in three women, Zika virus RNA was detected on multiple amniotic fluid samples, and one woman had negative Zika virus nucleic acid test results on multiple amniotic fluid samples. For the remaining six women, Zika virus RNA was initially detected in amniotic fluid, followed by a negative Zika virus nucleic acid test result on a subsequent amniotic fluid specimen collected later in gestation (range 1–18 weeks elapsed). There were no reports included in this review with a negative Zika virus nucleic acid test result on an amniotic fluid specimen followed by a positive nucleic acid test result on a later amniotic fluid specimen. Further clinical details of the nine mothers with a positive Zika virus nucleic acid test result and serial amniocenteses are presented in Table 4.
Table 4.
Clinical Details of Nine Mother-Fetus or Mother-Neonate Dyads Included in This Systematic Review With Serial Amniocenteses and Zika Virus-Positive Nucleic Acid Test Results on an Amniotic Fluid Specimen
| SR_ID (Reference) | Maternal ZIKV Infection, Weeks of Gestation | First AF ZIKV NAT-Positive Result |
2nd AF ZIKV NAT Result |
3rd AF ZIKV NAT Result |
Pregnancy Outcome | Neonatal or Fetal ZIKV Laboratory Results | Neonatal or Fetal Congenital Zika Syndrome Imaging and Clinical or Autopsy Examination Abnormalities |
|---|---|---|---|---|---|---|---|
| (Weeks Elapsed from Maternal Symptoms) | |||||||
| 234 Schaub et al29,30 | Symptomatic 1st trimester; ZIKV NAT-positive maternal blood | POS at 24 wk (10 or more) | POS at 28 wk (14 or more) | NEG at 38 (24 or more) | Live birth at 40 wk | ZIKV NAT-positive fetal blood, ZIKV NAT-negative neonatal CSF, urine, and blood | Clinical: microcephaly, excessive scalp skin. At 5 mo, severely abnormal vision and hearing by OAE, axial tonus hypotonia, spastic quadriparesy |
| 107 Driggers et al19 | Symptomatic at 12 wk; ZIKV NAT-positive maternal serum | POS at 20 wk (8) | POS at 21 wk (9)* | NA | Termination at 21 wk | ZIKV NAT-positive fetal tissues and placenta, membranes, umbilical cord | Autopsy: normal anatomy, cortical atrophy, corpus callosum not well visualized, no overt microscopic abnormalities of the eyes |
| 198 Rodo et al31 | Symptomatic at 8 wk; ZIKV NAT-positive maternal serum | POS at 19 wk (11) | NEG at 37 (29) | NA | Live birth at 37 wk | ZIKV NAT-negative urine, serum, CSF; ZIKV IgG positive serum and CSF at birth and 12 mo; ZIKV NAT-negative placenta, cord, and membranes | US and MRI: brain atrophy and polymicrogyria, mild ventriculomegaly with thinned corpus callosum, cortical and subcortical calcifications, microcephaly |
| 241 Schaub et al29,30 | Maternal serology suggests ZIKV infection between 9 and 19 wk† | POS at 32 wk (13 or more) | NEG at 34 (15 or more) | NA | Termination at 34 wk | ZIKV NAT-positive CSF, amnion, and fetal brain tissue; ZIKV NAT-negative fetal blood, placenta, ZIKV IgG positive fetal blood | Autopsy: microcephaly |
| 248 Suy et al23 | Symptomatic at 9 wk; ZIKV NAT-positive maternal serum | POS at UNK timing (UNK) | NEG at 37 (28) | NA | Live birth at 37 wk | ZIKV NAT-negative CSF, urine, serum, placenta, membranes, and umbilical cord | US and MRI: intracranial calcifications, cerebral atrophy, thinned corpus callosum, and microcephaly |
| 238 Schaub et al29,30 | Symptomatic at 10–12 wk; ZIKV NAT-positive maternal blood | POS at 24 wk (12–14) | NEG at 27 (15–17) | NA | Termination at 27 wk | ZIKV NAT-positive fetal brain tissue, ZIKV NAT-negative fetal blood, placenta, ZIKV IgG positive fetal blood | Autopsy: HC 3%, 1,145 g |
| 235 Schaub et al29,30 | Symptomatic at 10 wk; ZIKV NAT-positive maternal blood | POS at 19 wk (9) | POS at 22 (12) | NA | Termination at 22 wk | ZIKV NAT-positive fetal blood, placenta, fetal brain tissue, ZIKV IgG positive fetal blood | Autopsy: HC 5%, 375 g |
| 239 Schaub et al29,30 | Symptomatic at 11 wk; ZIKV NAT-positive maternal blood | POS at 26 wk (15) | POS at 30 (19) | NA | Termination at 30 wk | ZIKV NAT-positive CSF, fetal blood, fetal brain tissue, ZIKV IgG positive fetal blood, ZIKV NAT-negative placenta | Autopsy: HC 3%, 1,145g |
| 240 Schaub et al29,30 | Maternal serology suggests ZIKV infection between 7 and 9 wk‡ | POS at 20 wk (11 or more) | POS at 22 (13 or more) | NA | Termination at 22 wk | ZIKV NAT-positive CSF, fetal blood, placenta, amnion, fetal brain tissue | Autopsy: HC 3%, 315 g |
SR_ID, systematic review identification number; ZIKV, Zika virus; AF, amniotic fluid; NAT, nucleic acid test; POS, positive result on AF by NAT; NEG, negative result on AF by NAT; CSF, cerebrospinal fluid; OAE, otoacoustic emissions; IgG, immunoglobulin G; US, ultrasonography; MRI, magnetic resonance imaging; UNK, unknown; HC, head circumference.
Amniotic fluid result 2 performed at University of Helsinki using AF obtained at termination; AF sample obtained at termination was also sent to the Centers for Disease Control and Prevention and tested NEG for Zika virus NAT.
Infection between 9 and 19 weeks, estimated based on retrospective review of serologic testing, asymptomatic, ZIKV NAT—3rd trimester.
Infection between 7 and 9 weeks, estimated based on retrospective review of serologic testing, asymptomatic, ZIKV NAT—2nd trimester.
DISCUSSION
Congenital Zika syndrome abnormalities were confirmed in 78% (95% CI 72–84%) of pregnancies with prenatal congenital Zika syndrome brain findings and postnatal brain imaging or autopsy, suggesting that ultrasound examination can be valuable to detect these abnormalities. Microcephaly, cerebral atrophy, calcifications, and ventriculomegaly were more likely than not to be confirmed postnatally. Eye and limb findings detected by prenatal ultrasound examination were less likely to be confirmed at pregnancy completion.
Approximately one-fifth of prenatal congenital Zika syndrome findings were not confirmed postnatally; these may represent false positive results, transient findings, or incomplete postnatal evaluation or reporting. Interestingly, abnormal findings detected on prenatal ultrasound examination, although not always confirmed postnatally, may be associated with the detection of other congenital Zika syndrome-associated abnormalities.
Among 17% (95% CI 12–24%) of fetuses with normal prenatal ultrasound reports, congenital Zika syndrome structural abnormalities were detected at pregnancy completion, suggesting that prenatal ultrasound examination cannot always detect congenital Zika syndrome abnormalities, especially microcephaly. Detection of abnormalities by prenatal ultrasound examination depends on many factors, including timing of infection, timing of ultrasound examination, technical expertise, and severity of the abnormalities. As recommended by the CDC, pregnant women with Zika virus infection should consider serial prenatal ultrasound examination; more importantly, all neonates born to mothers with Zika virus infection during pregnancy should have a comprehensive clinical evaluation at birth, including a head ultrasound examination or other neuroimaging if clinically appropriate.11 It is concerning that only two-thirds of the fetuses in this review with a prenatal ultrasound finding consistent with a brain abnormality were reported to have any neuroimaging at birth, which may delay detection of abnormalities and initiation of clinical care tailored to the child’s needs.
Similar to other reports, our data illustrate a time lapse between onset of symptomatic maternal Zika virus infection and the appearance of congenital Zika syndrome findings on prenatal ultrasound examination (range 7–23 weeks). In a retrospective case series of serial head imaging of 17 fetuses with congenital Zika syndrome, Parra-Saavedra et al15 observed a median time elapsed from maternal infection to recognition of microcephaly on prenatal ultrasound examination of 18 weeks (range 15–24 weeks), with earlier detection of other anomalies (club foot, ventriculomegaly) in some patients. Based on the results of this systematic review, normal ultrasound examinations performed less than 10 weeks after a first-trimester symptomatic maternal infection did not provide adequate reassurance that a fetus was unaffected by Zika virus exposure. Further, among several pregnant women who underwent serial ultrasonography, fetal findings were not recognized on ultrasound examination by 20 weeks of gestation, the timeframe recommended for a comprehensive fetal anatomy screening.16 These results suggest that serial ultrasound examinations for fetal anatomy in the first trimester are likely of limited benefit, and serial ultrasound examination may be most useful during the second half of pregnancy. There are currently no known interventions to prevent congenital Zika syndrome after maternal Zika virus infection. At present, prenatal diagnosis of abnormalities can inform delivery planning to ensure the facility has specialized neonatal care, and to enable the family to prepare to care for a child with special needs.
One or more structural congenital Zika syndrome abnormalities were detected at pregnancy completion in similar proportions of women with positive and with negative Zika virus nucleic acid test amniocentesis results. The findings from these studies do not clarify the diagnostic role of Zika virus amniotic fluid testing for the detection of congenital Zika syndrome. Other factors such as the timing of infection, the timing of amniocentesis, Zika viral load, and maternal and fetal immune changes may influence the predictive value of amniocentesis. This review indicates that Zika virus RNA can be cleared from amniotic fluid during pregnancy; however, the exact mechanism for this clearance is unknown and additional study of the contribution of maternal and fetal immune clearance is warranted. Negative Zika virus test results on amniotic fluid specimens did not provide adequate reassurance that a fetus was unaffected by congenital Zika syndrome, particularly with increasing time elapsed between maternal infection and amniocentesis. Overall, many uncertainties remain regarding the role of Zika virus RNA testing of amniotic fluid for clinical management. Further study, including quantitative assessment of Zika virus RNA and comparison with other maternal and neonatal specimens might improve understanding of viral dynamics. It is of particular interest to explore potential clinical predictors of congenital Zika syndrome, such as viral load, severity of infection, and the role of maternal and fetal immune function.
This review is subject to several limitations. First, there is inherent heterogeneity in the population identified with microcephaly. For example, authors report several definitions of microcephaly. For this review, a neonate was included as microcephalic based on author report, despite possible differences in the methods used to evaluate fetal and neonatal head circumference across study sites and countries. Second, most articles did not report the results of maternal testing for other congenital infections known to cause birth defects (eg, cytomegalovirus and toxoplasmosis). Third, we included dyads that did not report laboratory testing, which was not widely available in all countries during the outbreak. These dyads were included if congenital Zika virus infection was suspected based on known epidemiologic link and fetal or neonatal abnormalities consistent with congenital Zika syndrome. Fourth, all studies in the review are observational, and subject to the known limitations of nonrandomized, noncomparative data sets. Finally, and most importantly, this review has inherent potential for publication bias; most included articles are case reports and case series, and likely represent the most severely affected neonates and fetuses. Thus, these findings should not be generalized. Further studies are needed to provide more insight into the predictive role of prenatal diagnostics for Zika virus infection.
Based on this review of published data, we conclude that prenatal ultrasound examination can detect structural findings associated with congenital Zika syndrome, though the absence of abnormal prenatal ultrasound findings does not preclude the possibility of congenital Zika syndrome. Detection of Zika virus RNA in amniotic fluid did not predict the risk for congenital Zika syndrome abnormalities in these cases. Although data are limited, clearance of Zika virus RNA from amniotic fluid appears possible after maternal infection; more research is needed to better understand how maternal and fetal immune function affects the risk of congenital Zika syndrome. Until more information becomes available, the decision to perform diagnostic testing for Zika virus infection should remain a shared decision between patients and clinical providers, and follow-up of children born to women with Zika virus infection during pregnancy remains critically important.
Supplementary Material
Acknowledgments
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Footnotes
Presented at ID Week, October 2–6, 2079, Washington DC, the Teratology Society Annual Meeting, June 22–26, 2019, San Diego, California, and Prenatal Diagnostics, June 6–9, 2018, Philadelphia, Pennsylvania.
The authors thank Onnalee Gomez, Centers for Disease Control and Prevention, for her assistance with designing and implementing this exhaustive literature search.
Financial Disclosure
The authors did not report any potential conflicts of interest.
REFERENCES
- 1.Moore CA, Staples JE, Dobyns WB, Pessoa A, Ventura CV, Fonseca EB, et al. Characterizing the pattern of anomalies in congenital Zika syndrome for pediatric clinicians. JAMA Pediatr 2017;171:288–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rasmussen SA, Jamieson DJ, Honein MA, Petersen LR. Zika virus and birth defects-reviewing the evidence for causality. New Engl J Med 2016;374:1981–7. [DOI] [PubMed] [Google Scholar]
- 3.Zorrilla CD, Garcia I, Garcia Fragoso L, De La Vega A. Zika virus infection in pregnancy: maternal, fetal, and neonatal considerations. J Infect Dis 2017;216(suppl_10):S891–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. CDC standard case definition for healthcare providers. 2018. Available at: https://www.cdc.gov/pregnancy/zika/research/technical-clinical.html#case-definition. Retrieved 8, 2018.
- 5.Soares de Oliveira-Szejnfeld P, Levine D, Melo AS, Amorim MM, Batista AG, Chimelli L, et al. Congenital brain abnormalities and Zika virus: what the radiologist can expect to see prenatally and postnatally. Radiology 2016;281:203–18. [DOI] [PubMed] [Google Scholar]
- 6.Vouga M, Baud D. Imaging of congenital Zika virus infection: the route to identification of prognostic factors. Prenatal Diagn 2016;36:799–811. [DOI] [PubMed] [Google Scholar]
- 7.Management of patients in the context of Zika virus. ACOG Committee Opinion No. 784. American College of Obstetricians and Gynecologists. Obstet Gynecol 2019;134:e64–70. [DOI] [PubMed] [Google Scholar]
- 8.Oduyebo T, Polen KD, Walke HT, Reagan-Steiner S, Lathrop E, Rabe IB, et al. Update: interim guidance for health care providers caring for pregnant women with possible Zika virus exposure-United States (including U.S. territories). Morb Mortal Wkly Rep 2017;66:781–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cytomegalovirus, parvovirus B19, varicella zoster, and toxo-plasmosis in pregnancy. Practice Bulletin No. 151. American College of Obstetricians and Gynecologists. Obstet Gynecol 2015;125:1510–25. [DOI] [PubMed] [Google Scholar]
- 10.Calvet G, Aguiar RS, Melo ASO, Sampaio SA, de Filippis I, Fabri A, et al. Detection and sequencing of Zika virus from amniotic fluid of fetuses with microcephaly in Brazil: a case study. Lancet Infect Dis 2016;16:653–60. [DOI] [PubMed] [Google Scholar]
- 11.Adebanjo T, Godfred-Cato S, Viens L, Fischer M, Staples JE, Kuhnert-Tallman W, et al. Update: interim guidance for the diagnosis, evaluation, and management of infants with possible congenital Zika virus infection—United States. Morb Mortal Wkly Rep 2017;66:1089–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol 2009;62:el–34. [DOI] [PubMed] [Google Scholar]
- 13.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol 2009;62:1006–12. [DOI] [PubMed] [Google Scholar]
- 14.Rice ME, Galang RR, Roth NM, Ellington SR, Moore CA, Valencia-Prado M, et al. Vital signs: zika-associated birth defects and neurodevelopmental abnormalities possibly associated with congenital Zika virus infection-U.S. territories and freely associated states, 2018. Morb Mortal Wkly Rep 2018; 67:858–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parra-Saavedra M, Reefhuis J, Piraquive JP, Gilboa SM, Badell ML, Moore CA, et al. Serial head and brain imaging of 17 fetuses with confirmed Zika virus infection in Colombia, south America. Obstet Gynecol 2017;130:207–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ultrasound in pregnancy. Practice Bulletin No. 175. American College of Obstetricians and Gynecologists. Obstet Gynecol 2016;128:e241–56. [DOI] [PubMed] [Google Scholar]
- 17.Benjamin I, Fernandez G, Figueira JV, Parpacen L, Urbina MT, Medina R. Zika virus detected in amniotic fluid and umbilical cord blood in an in vitro fertilization-conceived pregnancy in Venezuela. Fertil Steril 2017;107:1319–22. [DOI] [PubMed] [Google Scholar]
- 18.Brasil P, Pereira JP, Moreira ME, Nogueira RMR, Damasceno L, Wakimoto M, et al. Zika virus infection in pregnant women in Rio de Janeiro. N EnglJ Med 2016;375:2321–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brasil P, Pereira JP, Moreira ME, Nogueira RMR, Damasceno L, Wakimoto M, et al. Zika virus infection in pregnant women in Rio de Janeiro. N EnglJ Med 2016;375:2321–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mlakar J, Korva M, Tul N, Popovic M, Poljsak-Prijatelj M, Mraz J, et al. Zika virus associated with microcephaly. N Engl J Med 2016;374:951–8. [DOI] [PubMed] [Google Scholar]
- 21.Moron AF, Cavalheiro S, Milani H, Sarmento S, Tanuri C, de Souza FF, et al. Microcephaly associated with maternal Zika virus infection. BJOG 2016;123:1265–9. [DOI] [PubMed] [Google Scholar]
- 22.Perez S, Tato R, Cabrera JJ, Lopez A, Robles O, Paz E, et al. Confirmed case of Zika virus congenital infection, Spain, March 2016. Euro Surveill 2016. June 16;21. [DOI] [PubMed] [Google Scholar]
- 23.Suy A, Sulleiro E, Rodo C, Vazquez E, Bocanegra C, Molina I, et al. Prolonged Zika virus viremia during pregnancy. N Engl J Med 2016;375:2611–3. [DOI] [PubMed] [Google Scholar]
- 24.Vesnaver TV, Tul N, Mehrabi S, Parissone F, Strafela P, Mlakar J, et al. Zika virus associated microcephaly/micrencephaly—fetal brain imaging in comparison with neuropathology. BJOG 2017;124:521–5. [DOI] [PubMed] [Google Scholar]
- 25.Wemer H, Fazecas T, Guedes B, Lopes Dos Santos J, Daltro P, Tonni G, et al. Intrauterine Zika virus infection and microcephaly: correlation of perinatal imaging and three-dimensional virtual physical models. Ultrasound Obstet Gynecol 2016;47:657–60. [DOI] [PubMed] [Google Scholar]
- 26.Werner H, Sodre D, Hygino C, Guedes B, Fazecas T, Nogueira R, et al. First-trimester intrauterine Zika virus infection and brain pathology: prenatal and postnatal neuroimaging findings. Prenatal Diagn 2016;36:785–9. [DOI] [PubMed] [Google Scholar]
- 27.Sulleiro E, Frick MA, Rodo C, Espasa M, Thorne C, Espiau M, et al. The challenge of the laboratory diagnosis in a confirmed congenital Zika virus syndrome in utero: a case report. Medicine (Baltimore) 2019;98:e 15532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Valdespino-Vazquez MY, Sevilla-Reyes EE, Lira R, Yocupicio-Monroy M, Piten-Isidro E, Boukadida C, et al. Congenital Zika syndrome and extra-central nervous system detection of Zika virus in a pre-term newborn in Mexico. Clin Infect Dis 2019; 68:903–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.. Schaub B, Gueneret M, Jolivet E, Decatrelle V, Yazza S, Gueye H, et al. Ultrasound imaging for identification of cerebral damage in congenital Zika virus syndrome: a case series. Lancet Child Adolesc Health 2017;1:45–55. [DOI] [PubMed] [Google Scholar]
- 30.Schaub B, Vouga M, Najioullah F, Gueneret M, Monthieux A, Harte C, et al. Analysis of blood from Zika virus-infected fetuses: a prospective case series. Lancet Infect Dis 2017; 17: 520–7. [DOI] [PubMed] [Google Scholar]
- 31.Rodo C, Suy A, Sulleiro E, Soriano-Arandes A, Anton A, Garcia I, et al. In utero negativization of Zika virus in a case with serious Central Nervous System abnormalities. Clin Microbiol Infect 2017;24:549.el–3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


