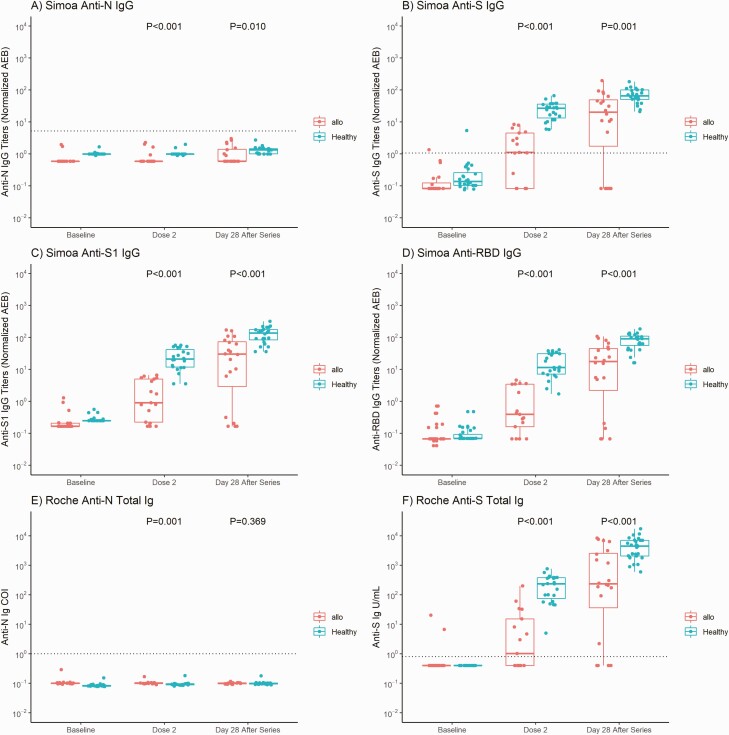
Figure 1.
Anti–severe acute respiratory syndrome coronavirus 2 antibody results for allogeneic hematopoietic stem cell transplant (HSCT) recipients and healthy participants. A–D, Simoa assay titers for anti-N immunoglobulin (Ig) G (A), anti-S IgG (B), anti-S1 IgG (C), and anti–receptor-binding domain (RBD) IgG. E, F, Roche assay results for anti-N (E) and anti-S (F) total immunoglobulin (Ig) values at baseline (before vaccination), at dose 2, and 28 days after completion of the vaccine series. Thick horizontal bars and error lines represent medians and interquartile ranges, respectively, and dotted lines represent cutoff thresholds for each assay (Simoa assay: 1.07 normalized average enzymes per bead [AEB] for anti-S IgG and 5.2 normalized AEB for anti-N IgG [no thresholds determined for anti-RBD or anti-S1]; Roche assay: 0.8 U/mL for anti-S and 1.0 cutoff index [COI] for anti-N total immunoglobulin). All participants in the allogeneic HSCT and healthy cohorts had negative anti-N IgG (A) and anti-N total immunoglobulin (E) results throughout the study. P values represent comparisons between the allogeneic HSCT and healthy cohorts at dose 2 and 28 days after series completion. All temporal values within the allogeneic HSCT or healthy cohort for anti-S, anti-S1, and anti-RBD (Simoa) and anti-S (Roche) represent significant differences (P < .001), indicating a significant rise in antibody magnitude from baseline to the time of dose 2, and from dose 2 to 28 days after series completion.