Table 5.
Interactions of anticoagulant drugs with experimental COVID-19 therapies
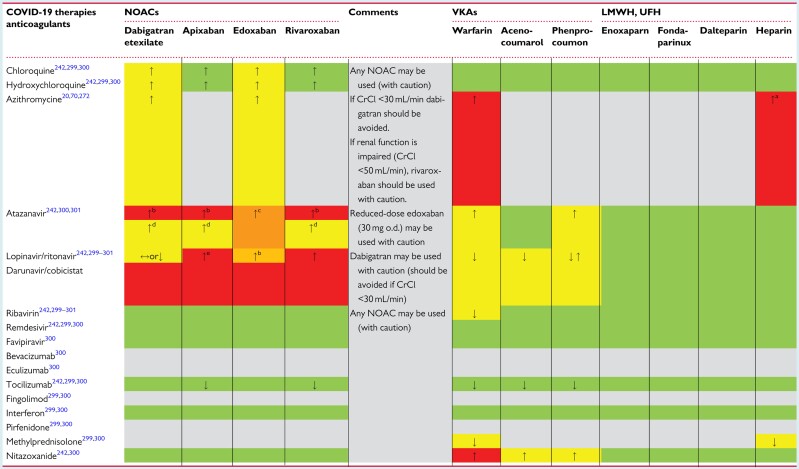
|
Light grey colour: no information found. Green colour: no clinically significant interaction is expected, or potential interaction is likely to be of weak intensity, not requiring additional action/monitoring or dose adjustment. Yellow colour: potential interaction which may require additional monitoring (e.g. more frequent INR monitoring if on VKAs). Orange colour: potential interaction which may require a dose adjustment. Red colour: the drugs should not be co-administered. ↑, potential increased exposure to the anticoagulant drug; ↓, potential decreased exposure to the anticoagulant drug; ↔, no significant effect on the exposure to the drug.
COVID-19, coronavirus disease 2019; CrCl, creatinine clearance; LMWH, low molecular weight heparin; NOACs, non-vitamin K antagonist oral anticoagulants; o.d., once daily; UFH, unfractionated heparin; VKAs, vitamin K antagonists.
Azithromycin increases the effect of heparin by decreasing its metabolism.300
There is an overall agreement that the use of NOACs is not recommended when atazanavir is given in combination with its enhancers, ritonavir or cobicistat.
The EMA product label for edoxaban advises the consideration of dose reduction from 60 mg once daily to 30 mg once daily with concomitant use of strong P-glycoprotein inhibitors.
No data on the safety/efficacy of use of NOACs when co-administered with atazanavir are known; if their use is deemed indicated, one should consider monitoring plasma level of the NOACs in this unknown condition, in line with the recommendation that was made in the last EHRA Practical Guide.298
The US product label for apixaban proposes the use of apixaban at reduced dose (2.5 mg twice daily) if needed.
