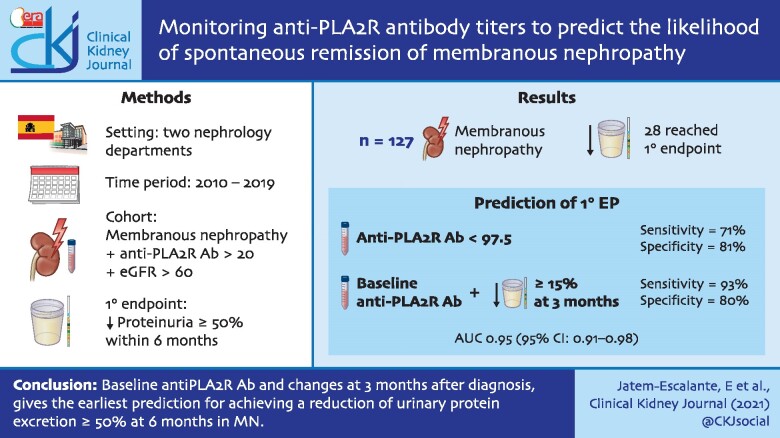
ABSTRACT
Background
In anti-phospholipase A2 receptor (PLA2R) membranous nephropathy (MN) there is controversy whether spontaneous remission (SR) can be predicted using a single titre or by assessing the dynamic changes in anti-PLA2R antibody (ab) titres. The study objective was to identify the optimal dynamics of anti-PLA2Rab titres to predict SR in MN.
Methods
A total of 127 nephrotic patients with anti-PLA2R-MN were prospectively followed up for 6 months under conservative treatment. Anti-PLA2Rabs and proteinuria were assessed at diagnosis and monthly thereafter. The primary endpoint (PEP) was a reduction of proteinuria ≥50% at 6 months. Logistic models with baseline and evolutive anti-PLA2Rab titres were developed to predict the PEP.
Results
A total of 28 patients (22%) reached the PEP. These patients were more frequently female and had significantly lower baseline proteinuria and anti-PLA2Rab titres. An anti-PLA2R titre ≤97.5 RU/mL at diagnosis had a sensitivity of 71% and a specificity of 81% to predict the PEP. The model including baseline anti-PLA2Rabs and a reduction ≥15% at 3 months predicted the PEP with a sensitivity of 93% and a specificity of 80%, with an area under the curve that was significantly greater than that obtained with relative changes of proteinuria in the same period of time {odds ratio [OR] 0.95 [95% confidence interval (CI) 0.91–0.98 versus OR 0.79 [95% CI 0.70–0.88], respectively; P = 0.0013}.
Conclusions
Combining the baseline anti-PLA2Rab titres with their relative changes at 3 months after diagnosis gives the earliest prediction for achieving a reduction of urinary protein excretion ≥50% at 6 months in MN, thereby shortening the observation period currently recommended to make individualized decisions to start immunosuppressive therapy.
Keywords: anti-PLA2R antibodies, membranous nephropathy, nephrotic syndrome, prediction, spontaneous remission
Graphical Abstract
Graphical Abstract.
INTRODUCTION
Membranous nephropathy (MN) is the main cause of idiopathic nephrotic syndrome in adults [1–3]. Approximately 75% of primary MN cases are due to renal deposits of immunoglobulin G4 (IgG4) antibodies against the M-type phospholipase A2 receptor (PLA2R) expressed in the podocytes [4, 5]. If left untreated, about one-third of MN patients achieved spontaneous remission (SR), while a similar percentage may progress to chronic renal failure [3, 6, 7]. Given that a high percentage of patients may present with SR, immunosuppressive treatment is reserved for those patients with a low probability of SR, for those cases in which nephrotic syndrome is associated with a high risk of serious complications or when there is a high risk of progressive loss of renal function [8–10]. The current guidelines indicate that by categorizing patients as low, moderate, high and very high risk of progressive loss of kidney function allows one to identify those who are more likely to develop SR within ˂24 months [11]. For patients in the medium- or high-risk categories, it is considered reasonable to perform a follow-up under treatment with angiotensin II blockers and to initiate immunosuppressive treatment depending on the evolution of proteinuria 6 months after diagnosis. The explicit criterion specified in the Kidney Disease: Improving Global Outcomes (KDIGO) guidelines for starting immunosuppressive treatment is the absence of a reduction ≥50% in urinary protein excretion at 6 months, due to its value as the best surrogate variable related to the probability of SR [11, 12]. Anti-PLA2R antibody (ab) titres have been identified as potential biomarker candidates for therapeutic decision making because they correlate with disease activity [9, 13–17]. The KDIGO guidelines indicate that PLA2Rab should be measured at different times after diagnosis because changes in PLA2Rab levels during follow-up likely add to risk estimation. Since the disappearance of PLA2Rab precedes clinical remission [15–22], our hypothesis was that monitoring antibody titres after diagnosis could be useful for early identification of those patients who will meet the criterion of 50% reduction in proteinuria at 6 months, thus allowing shortening of the observation period necessary to identify patients with a higher probability of SR.
The aim of our study is to analyse whether monitoring the kinetics of PLA2Rab titres after diagnosis is useful for the early identification of patients who will achieve a reduction in proteinuria ≥50% within the first 6 months after diagnosis.
MATERIALS AND METHODS
Study design and patients
This prospective cohort study included patients newly diagnosed with nephrotic syndrome secondary to anti-PLA2R-associated MN between 2010 and 2019 in two referral nephrology departments. The inclusion criteria were MN associated with anti-PLA2R confirmed by renal biopsy, an estimated glomerular filtration rate (eGFR) >60 mL/min/1.73 m2 at the time of diagnosis and anti-PLA2Rab titres >20 RU/mL. Exclusion criteria were uncontrollable nephrotic syndrome, evidence of thrombotic complications or rapid deterioration of kidney function defined by a decrease in eGFR ≥25% from baseline not attributable to external factors, denial to give their written consent and loss of follow-up within the first 6 months after diagnosis.
The study was conducted according to the Declaration of Helsinki for medical research involving human subjects and the study protocol was approved by the local independent ethics committee. All patients gave their written consent to participate in the study.
Monitoring and treatment protocol
Observation period after diagnosis
Patients were assessed at diagnosis (baseline) and followed for 6 months with monthly clinical and analytical assessment. Demographic and clinical data recorded at baseline included age, gender, diagnosis of diabetes mellitus (DM), serum albumin, creatinine, total cholesterol, anti-PLA2R, urine protein and eGFR. During the 6-month observation period, all patients were treated with diuretics, statins and renin–angiotensin system blockers and no patient received immunosuppressive treatment.
Outcome measure and operational definitions
To maintain coherence with the criteria defined in the KDIGO guidelines [11], the primary endpoint (PEP) was a reduction of proteinuria ≥50% within the first 6 months after diagnosis.
Nephrotic syndrome was defined by the presence of oedema, albuminaemia <3.5 g/dL and 24-h proteinuria ≥3.5 g/1.73 m2 of body surface area, with or without dyslipidaemia [11]. Complete clinical remission was considered as proteinuria ≤0.3 g/24 h with albuminaemia >3.5 g/dL and partial clinical remission was defined as proteinuria between 0.3 and 3.5 g/day and albuminaemia >3.5 g/dL.
Follow-up after the 6-month observation period
After the 6-month observation period, patients who met the PEP were followed up under symptomatic treatment and patients who did not meet the PEP started immunosuppressive treatment according to the guidelines.
Measurements and laboratory methods
Anti-PLA2Rab levels were assessed by enzyme-linked immunosorbent assasy (Euroimmun, Lübeck, Germany; linearity 6–1500 RU/mL, lower limit of detection 0.6 RU/mL) [16, 23]. Intra-assay precision was obtained by analysing five patient samples with known concentrations five times on the same plate, which was 3.9% (mean 30 U/mL) at low concentrations and 5.8% (mean 350 U/mL) at high concentrations. Interassay precision was obtained by analysing two patient samples with known concentrations in 10 separate assays, which was 4.8% (mean 35 U/mL) at low concentrations and 7% (mean 250 U/mL) at high concentrations. All determinations for anti-PLA2Rab were performed by investigators who were blinded to the clinical status of patients. To avoid variability between the different assays, all samples from the same individual were analysed using the same plate. Serum creatinine was measured by a traceable isotope dilution mass spectrometry compensated method (Hitachi Modular P-800, Roche Diagnostics, Berlin, Germany). eGFR was calculated using the Chronic Kidney Disease Epidemiology Collaboration equation [24]. Measurements were performed in each patient at diagnosis and monthly during the observation period.
Statistical analysis
Continuous variables were expressed as mean (SD) or as median and quartiles. Categorical variables were described as frequencies and proportions. The differences in proportions between groups were analysed using Fisher’s exact test or the chi-square test. The changes in anti-PLA2R titres and 24-h proteinuria at 3, 4, 5 and 6 months (Δ anti-PLA2R; Δ proteinuria) were calculated as the relative difference between those periods and baseline values. The evolution of antibody titres during the observation period was explored using an analysis of variance for repeated measurements. Between-group differences in quantitative variables were analysed using the Student’s t-test or its non-parametric counterpart, Wilcoxon rank sum test for non-normal variables. Multivariable analysis to identify the variables associated with the outcome measure was assessed by a logistic regression model. To assess changes in anti-PLA2R titres and proteinuria during the follow-up period while accounting for the correlation between the repeated measurements of each patient, we used linear mixed effects models. Models included subjects as random effects and group (early remission), time and the interaction of time–group as fixed effects. The difference of trend over time between groups was assessed by evaluating the interaction term. The performance of anti-PLA2R titres and proteinuria-based models at baseline and at different times after diagnosis and their relative changes from basal values at those periods was assessed using receiver operating characteristics (ROC) curves.
Statistical analyses were conducted using R version 3.6 (R Foundation for Statistical Computing, Vienna, Austria) and SPSS version 20.0 (IBM, Armonk, NY, USA) and MedCalc (Ostend, Belgium) statistical software packages.
RESULTS
Patient characteristics and outcomes
Overall, 148 patients were initially evaluated for the study and 21 were excluded due to one of the following reasons: not giving their informed consent to participate [n = 8 (5.4%)], having criteria for immediate immunosuppressive treatment due to deep vein thrombosis [n = 4 (2.7%)] and transfer to another centre with no possibility of obtaining follow-up clinical data [n = 9 (6%)]. The final study cohort included 127 patients.
Of the 127 patients, 28 (22%) achieved the PEP. All these patients subsequently achieved SR, 5 (3.9%) complete clinical remission and 23 (18.1%) partial clinical remission in a mean time of 7.2 months (SD 2.3) after the PEP. Table 1 summarizes the demographic and clinical characteristics of patients at baseline, grouped according to achievement of the PEP. At the time of diagnosis, patients who achieved the PEP showed significantly lower proteinuria and anti-PLA2Rab titres than patients not meeting the PEP. Males were more frequent among the patients not achieving PEP. There were no significant differences in any of the other variables between these two groups. On univariate analysis, baseline proteinuria {odds ratio [OR] 0.67 [95% confidence interval (CI) 0.54–0.82]; P < 0.001} and anti-PLA2R titres [OR 0.98 (95% CI 0.97–0.99)] were associated with the PEP.
Table 1.
Demographic and clinical characteristics of patients at baseline with and without PEP
| Variable | PEP (n = 28) | No PEP (n = 99) | P-value |
|---|---|---|---|
| Male, n (%) | 8 (28.6) | 49 (49.5) | 0.04 |
| Age (years), mean (SD) | 53.1 (16.4) | 55.8 (16) | 0.41 |
| Time from clinical onset to kidney biopsy (weeks), median (IQR) | 4.9 (3–6.5) | 4.5 (3.5–6.9) | 0.39 |
| DM, n (%) | 2 (7.1) | 5 (5.1) | 0.66 |
| Thromboprophylaxis, n (%) | 27 (96.4) | 89 (89.9) | 0.27 |
| RAAS blockade, n (%) | 24 (85.7) | 85 (85.9) | 0.98 |
| Creatinine (mg/dL), mean (SD) | 0.9 (0.2) | 0.87 (0.3) | 0.71 |
| eGFR (mL/min), mean (SD) | 90.3 (29) | 94.9 (22.1) | 0.35 |
| Proteinuria (g/24 h), mean (SD) | 8.3 (1.8) | 10.9 (2.7) | <0.001 |
| Albuminaemia (g/dL), mean (SD) | 2.4 (0.5) | 2.3 (0.4) | 0.13 |
| Total cholesterol (mg/dL), mean (SD) | 305.9 (53.9) | 293.4 (72.3) | 0.39 |
| Anti-PLA2R (rU/mL), mean (SD) | 94.4 (52.8) | 162.4 (91.14) | <0.001 |
RAAS, renin–angiotensin–aldosterone system. Significant values are in bold.
Predictive value of the kinetics of anti-PLA2Rab titres
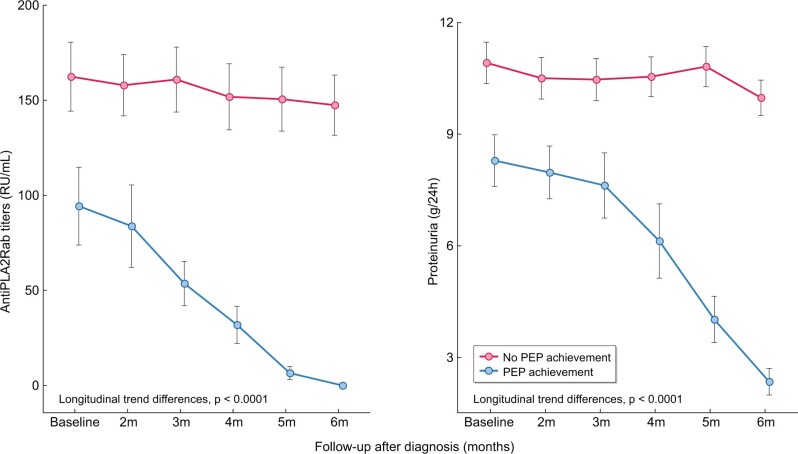
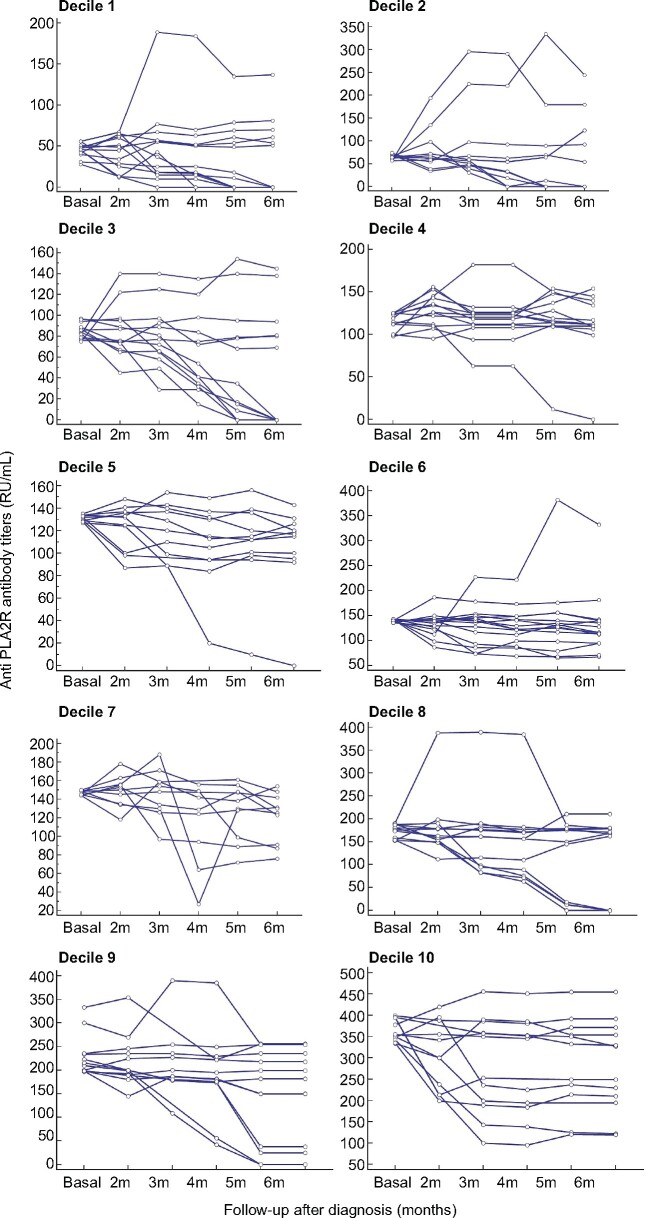
Figure 1 and Table 2 show the evolution of anti-PLA2Rab titres and proteinuria through the observation period of 6 months in patients who achieved the PEP and in patients who did not achieve the PEP. The patients achieving the PEP had a significant reduction of both anti-PLA2Rab titres and proteinuria over time, with more pronounced slopes and longitudinal trend differences compared with patients who did not achieve the PEP (P < 0.001). The patients reaching the PEP were negative for serum anti-PLA2Rab and presented a mean 24-h proteinuria of 2.4 ± 0.92 g (Table 2) and a mean serum albumin of 4.1 ± 0.2 g/dL, with resolution of nephrotic syndrome. Figure 2 shows the graphic representation of the evolution of anti-PLA2Rab titres of patients classified according to baseline quartiles of anti-PLA2Rab titres. In all groups, three different evolutive kinetics were observed, including patients with stable titres, patients with negative slopes and patients with positive slopes. An anti-PLA2R cut-off of ≤97.5 RU/mL, measured at diagnosis, predicted the PEP with a sensitivity of 71% and a specificity of 81%. Predictive models for the PEP were developed based on anti-PLA2R titres and proteinuria at baseline and with their relative changes at 2, 3, 4 and 5 months. The results of these models are shown in Tables 3 and 4. The earliest and better performance to predict the PEP was obtained including the baseline values and the relative changes in anti-PLA2Rab titres at 3 months. The area under the curve (AUC) of this model was significantly greater than that obtained with the model based on the relative changes of proteinuria in the same period of time [AUC = 0.95 (95% CI 0.91–0.98) versus 0.79 (0.7–0.88), respectively; P = 0.0013] (Figure 3).
FIGURE 1:
Evolution of anti-PLA2Rab titres and proteinuria among patients according to achievement of the PEP. The values are expressed as mean ± SD with a 95% CI.
Table 2.
Evolution of anti-PLA2Rab titres and 24-h proteinuria during the 6-month observation period in patients with or without PEP
| Variable | PEP | No PEP | P-value |
|---|---|---|---|
| Anti-PLA2R (rU/mL), mean (SD) | |||
| 2 months | 83.8 (55.8) | 157.3 (80.2) | <0.001 |
| 3 months | 53.7 (29.6) | 160.9 (84.3) | 0.002 |
| 4 months | 31.9 (25.19) | 151.6 (85.7) | <0.001 |
| 5 months | 6.5 (8.85) | 150.6 (83.9) | <0.001 |
| 6 months | 0 | 147.4 (79.3) | <0.001 |
| Proteinuria (g/24 h), mean (SD) | |||
| 2 months | 7.9 (1.8) | 10.5 (2.8) | 0.02 |
| 3 months | 7.6 (2.25) | 10.4 (2.7) | <0.001 |
| 4 months | 6.1 (2.57) | 10.5 (2.62) | <0.001 |
| 5 months | 4.02 (1.6) | 10.8 (2.7) | <0.001 |
| 6 months | 2.4 (0.92) | 9.9 (2.37) | <0.001 |
Significant values in bold.
FIGURE 2:
Evolution of the anti-PLA2Rab titres of each patient during the observation period of 6 months after diagnosis. Each one of the graphs represents the evolution of the antibody titres of each patient throughout the observation period of 6 months. The total group has been divided into deciles, based on the baseline anti-PLA2Rab titres, to allow visualization of the evolution of the antibody titres in each of them.
Table 3.
Logistic regression model based on anti-PLA2R titres to predict the PEP
| Anti-PLA2R | OR (95% CI) | P-value | AUC (95% CI) |
|---|---|---|---|
| Baseline | 0.98 (0.97–0.99) | <0.001 | 0.75 (0.64–0.86) |
| Baseline | 0.98 (0.97–0.99) | <0.001 | 0.79 (0.69–0.9) |
| Δ at 2 monthsa | 0.97 (0.95–0.99) | 0.003 | – |
| Baseline | 0.94 (0.91–0.97) | <0.001 | 0.95 (0.91–0.98) |
| Δ at 3 monthsa | 0.93 (0.89–0.96) | <0.001 | |
| Baseline | 0.93 (0.9–0.96) | <0.001 | 0.95 (0.94–1) |
| Δ at 4 monthsa | 0.92 (0.88–0.95) | <0.001 | – |
| Baseline | 0.87 (0.8–0.95) | <0.001 | 0.99 (0.98–1) |
| Δ at 5 monthsa | 0.88 (0.81–0.95) | <0.001 | – |
Δ: relative difference between value at each month and baseline value.
Table 4.
Logistic regression model–based proteinuria to predict the PEP
| Proteinuria | OR (95% CI) | P-value | AUC (95% CI) |
|---|---|---|---|
| Baseline | 0.67 (0.56–0.82) | <0.001 | 0.79 (0.71–0.87) |
| Baseline | 0.67 (0.54–0.82) | <0.001 | 0.78 (0.7–0.86) |
| Δ at 2 monthsa | 0.82 (0.47–1.42) | 0.47 | – |
| Baseline | 0.65 (0.53–0.8) | <0.001 | 0.79 (0.7–0.88) |
| Δ at 3 monthsa | 0.64 (0.41–1.01) | 0.05 | |
| Baseline | 0.53 (0.4–0.71) | <0.001 | 0.89 (0.83–0.96) |
| Δ at 4 monthsa | 0.48 (0.33–0.69) | <0.001 | – |
| Baseline | 0.33 (0.17–0.65) | 0.001 | 0.99 (0.98–1) |
| Δ at 5 monthsa | 0.16 (0.06–0.4) | <0.001 | – |
Δ: relative difference between value at each month and baseline value.
FIGURE 3:
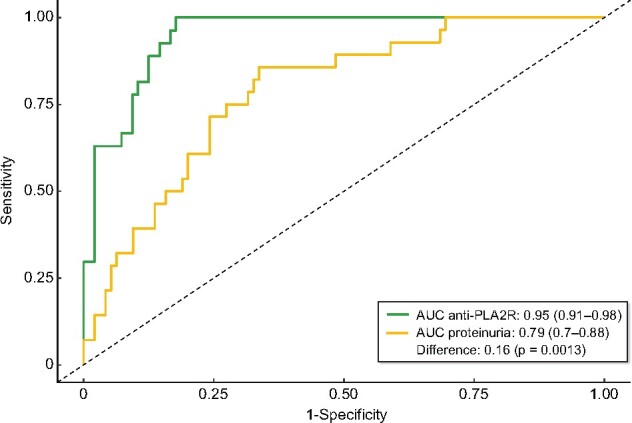
ROC curves comparing the predictive models based on anti-PLA2Rab titres and proteinuria at baseline and at 3 months.
DISCUSSION
Classical prediction models for SR in MN are based mainly on GFR and 24-h proteinuria [11]. Since the change in anti-PLA2R titre precedes the change in proteinuria and clinical status [13, 15–18], we hypothesized that a model based on anti-PLA2R titres could allow early identification of patients who will show the PEP of at least a 50% reduction in proteinuria within the first 6 months after diagnosis, which is currently considered the best surrogate measure of the likelihood of SR [9, 10]. The results of our study indicate that measuring anti-PLA2Rab titres at baseline and at 3 months allowed accurate early identification of patients who subsequently achieved the PEP, which in all cases was followed by SR. This identification could be done before any significant change in urinary protein excretion was observed and outperformed the prediction obtained from changes in urinary protein excretion, making it possible to shorten the observation period needed to evaluate the outcome measure defined by the KDIGO guidelines [11].
Despite the recent efforts to find optimal cut-off values of anti-PLA2Rab to predict SR, there is no consensus regarding the optimal value—or range of values—and the time at which the antibody should be measured. A recent study [25] found that antibody titres ˂40 U/mL at the time of diagnosis strongly indicated the likelihood of SR in the first 6 months based on a retrospective observation of a cohort of MN patients. Likewise, other retrospective studies gave specific antibody levels able to predict SR. However, immunological and clinical criteria in real-life practice tend to vary among centres, thereby precluding the possibility of drawing strong conclusions regarding the predictive ability of each cut-off [9, 14, 15]. Certainly some of these models for SR prediction are based on anti-PLA2R titre measurements at a single time period (at diagnosis), which constitutes a limitation. In our study, a cut-off concentration of anti-PLA2R ≤97.5 RU/mL at baseline predicted the PEP at 6 months with a sensitivity of 71% and a specificity of 81%. This means that nearly one-fourth of patients who achieved the PEP had baseline anti-PLA2R titres above this cut-off. Furthermore, 20% of the patients with anti-PLA2R values below this cut-off at baseline failed to achieve PEP.
In previous studies [18, 26], it was found that patients with anti-PLA2Rab titres in the high tertile or with absolute values >275 U/mL have a probability of SR close to 20%. In our cohort of patients, none of the patients with baseline titres at these levels reached the PEP at 6 months and in all cases, immunosuppressive treatment was started. These data do not exclude the possibility that some of them could have achieved SR if the observation period had been longer than is currently recommended in the guidelines. When combining the baseline levels with the reduction in the anti-PLA2R titres at 3 months, the model predicted the PEP with better sensitivity and specificity (93% and 80%, respectively) than the baseline titres. These findings indicate that, besides baseline titres, antibody kinetics during follow-up should be considered to predict the PEP. The rationale of combining the baseline and the evolutive titres is supported by the data shown in Figure 2 in which it can be observed that any baseline value of anti-PLA2R titre is just a single measure corresponding to a distinct kinetic pattern that modifies the predictive value of the baseline titres. Among all possible combinations of baseline titres and evolutive changes at fixed time periods, the earliest predictive model of the PEP was obtained with the relative changes at 3 months.
The main strengths of our study are the prospective design, with explicit intervention criteria common to all patients, and that we analysed the most widely used enzyme-linked immunoassay technique in clinical practice for the measurement of anti-PLA2Rab titres. The main limitations are the absence of external validation and the limitation of the observation period without treatment to 6 months, which was chosen in order to meet the criteria recommended in most guidelines for starting immunosuppressive therapy. However, longer watching periods should be considered in future studies, as they may allow identification of patients with no clinical signs of deterioration who would achieve SR later. Of note, our results are applicable only to patients with nephrotic syndrome and without renal failure at the time of diagnosis; the performance of anti-PLA2R titres as biomarkers in patients who do not meet these criteria cannot be inferred.
In summary, our study provides clinicians with a model that, by combining the baseline anti-PLA2Rab titres and their relative changes along the first 3 months after diagnosis, allows an accurate estimation of the likelihood of achieving the endpoint of a reduction in urinary protein excretion ≥50% at 6 months, which is currently considered to be the best surrogate measure of the probability of achieving SR. This model allows shortening of the observation period, thus contributing to individualized decisions to start immunosuppressive therapy.
FUNDING
No additional funding was obtained in the development of this study.
AUTHORS’ CONTRIBUTIONS
J.E.E. and S.M.A. were responsible for the research idea and study design. J.E.E., M.C.M.L., C.L., P.A. and M.C. were responsible for data acquisition. J.E.E., S.M.A. and M.M.M. were responsible for data analysis/interpretation. S.M.A., E.G.L., I.D.B. and J.E.E. were responsible for statistical analysis. S.M.A. and M.C.M.L. were responsible for supervision or mentorship. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
CONFLICT OF INTEREST STATEMENT
None of the authors have any conflicts of interest to declare.
Contributor Information
Elias Jatem-Escalante, Servicio de Nefrología, Hospital Universitario Arnau de Vilanova, Lleida, Spain; Institut de Recerca Biomèdica, Lleida, Spain.
María Luisa Martín-Conde, Servicio de Nefrología, Hospital Universitario Arnau de Vilanova, Lleida, Spain; Institut de Recerca Biomèdica, Lleida, Spain.
Esther Gràcia-Lavedan, Institut de Recerca Biomèdica, Lleida, Spain; Centro de Investigación Biomédica en Red de Enfermedades Respiratorias (CIBERES), Madrid, Spain.
Ivan D Benítez, Institut de Recerca Biomèdica, Lleida, Spain; Centro de Investigación Biomédica en Red de Enfermedades Respiratorias (CIBERES), Madrid, Spain.
Jorge Gonzalez, Servicio de Nefrología, Hospital Universitario Arnau de Vilanova, Lleida, Spain.
Laura Colás, Institut de Recerca Biomèdica, Lleida, Spain.
Alicia Garcia-Carrasco, Institut de Recerca Biomèdica, Lleida, Spain.
Cristina Martínez, Institut de Recerca Biomèdica, Lleida, Spain.
Alfons Segarra-Medrano, Servicio de Nefrología, Hospital Universitario Arnau de Vilanova, Lleida, Spain; Institut de Recerca Biomèdica, Lleida, Spain; Vall d’Hebrón Institut de Recerca, Barcelona, Spain.
REFERENCES
- 1. Tiebosch ATMG, Wolters J, Frederik PFM et al. Epidemiology of idiopathic glomerular disease: a prospective study. Kidney Int 1987; 32: 112–116 [DOI] [PubMed] [Google Scholar]
- 2. Kerjaschki D. Pathomechanisms and molecular basis of membranous glomerulopathy. Lancet 2004; 364: 1194–1196 [DOI] [PubMed] [Google Scholar]
- 3. Donadio JV, Torres VE, Velosa JA et al. Idiopathic membranous nephropathy: the natural history of untreated patients. Kidney Int 1988; 33: 708–715 [DOI] [PubMed] [Google Scholar]
- 4. Beck LH, Salant DJ. Membranous nephropathy: from models to man. J Clin Invest 2014; 124: 2307–2314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Beck LH, Bonegio RGB, Lambeau G et al. M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med 2009; 361: 11– 21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fulladosa X, Praga M, Segarra A et al. Glomerulonefritis membranosa. Nefrología 2007; 27: 70 [Google Scholar]
- 7. Polanco N, Gutierrez E, Rivera F et al. Spontaneous remission of nephrotic syndrome in membranous nephropathy with chronic renal impairment. Nephrol Dial Transplant 2012; 27: 231–234 [DOI] [PubMed] [Google Scholar]
- 8. Howman A, Chapman TL, Langdon MM et al. Immunosuppression for progressive membranous nephropathy: a UK randomised controlled trial. Lancet 2013; 381: 744–751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pei Y, Cattran D, Greenwood C. Predicting chronic renal insufficiency in idiopathic membranous glomerulonephritis. Kidney Int 1992; 42: 960–966 [DOI] [PubMed] [Google Scholar]
- 10. Ronco P, Debiec H. Membranous glomerulopathy: the evolving story. Curr Opin Nephrol Hypertens 2010; 19: 254–259 [DOI] [PubMed] [Google Scholar]
- 11.Kidney Disease: Improving Global Outcomes Glomerulonephritis Work Group. KDIGO clinical practice guidelines for glomerulonephritis. Kidney Int Suppl 2012; 2: 139–274 [Google Scholar]
- 12. van den Brand JA, Hofstra JM, Wetzels JF. Prognostic value of risk score and urinary markers in idiopathic membranous nephropathy. Clin J Am Soc Nephrol 2012; 7: 1242–1248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cattran DC, Pei Y, Greenwood CM et al. Validation of a predictive model of idiopathic membranous nephropathy: its clinical and research implications. Kidney Int 1997; 51: 901–907 [DOI] [PubMed] [Google Scholar]
- 14. Oh YJ, Yang SH, Kim DK et al. Autoantibodies against phospholipase A2 receptor in Korean patients with membranous nephropathy. PLoS One 2013; 8: e62151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wei SY, Wang YX, Li JS et al. Serum anti-PLA2R antibody predicts treatment outcome in idiopathic membranous nephropathy. Am J Nephrol 2016; 43: 129–140 [DOI] [PubMed] [Google Scholar]
- 16. Hofstra JM, Beck LH, Beck DM et al. Anti-phospholipase A2 receptor antibodies correlate with clinical status in idiopathic membranous nephropathy. Clin J Am Soc Nephrol 2011; 6: 1286–1291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Seitz-Polski B, Debiec H, Rousseau A et al. Phospholipase A2 receptor 1 epitope spreading at baseline predicts reduced likelihood of remission of membranous nephropathy. J Am Soc Nephrol 2018; 29: 401–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hofstra JM, Debiec H, Short CD et al. Antiphospholipase A2 receptor antibody titer and subclass in idiopathic membranous nephropathy. J Am Soc Nephrol 2012; 23: 1735–1743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Segarra-Medrano A, Jatem-Escalante E, Carnicer-Cáceres C et al. Evolution of antibody titre against the M-type phospholipase A2 receptor and clinical response in idiopathic membranous nephropathy patients treated with tacrolimus. Nefrologia 2014; 34: 491–497 [DOI] [PubMed] [Google Scholar]
- 20. Medrano AS, Escalante EJ, Cáceres CC et al. Prognostic value of the dynamics of M-type phospholipase A2 receptor antibody titers in patients with idiopathic membranous nephropathy treated with two different immunosuppression regimens. Biomarkers 2015; 20: 77–83 [DOI] [PubMed] [Google Scholar]
- 21. Timmermans SAMEG, Abdul Hamid MA, Cohen Tervaert JW et al. Anti-PLA2R antibodies as a prognostic factor in PLA2R-related membranous nephropathy. Am J Nephrol 2015; 42: 70–77 [DOI] [PubMed] [Google Scholar]
- 22. De Vriese AS, Glassock RJ, Nath KA et al. A proposal for a serology-based approach to membranous nephropathy. J Am Soc Nephrol 2017; 28: 421–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Behnert A, Schiffer M, Müller-Deile J et al. Antiphospholipase A2 receptor autoantibodies: a comparison of three different immunoassays for the diagnosis of idiopathic membranous nephropathy. J Immunol Res 2014; 2014: 143274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Levey AS, Stevens LA, Schmid CH et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150: 604–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rodas LM, Matas-García A, Barros X et al. Antiphospholipase 2 receptor antibody levels to predict complete spontaneous remission in primary membranous nephropathy. Clin Kidney J 2018; 12: 36–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rousseau A. Sub-analysis of data from GEMRITUX cohort. Personal communication received by Wetzels JF, 15 January 2019; 146.