Fig. 4. Role of electrostatic interactions in the compartmentalization of peripheral membrane proteins.
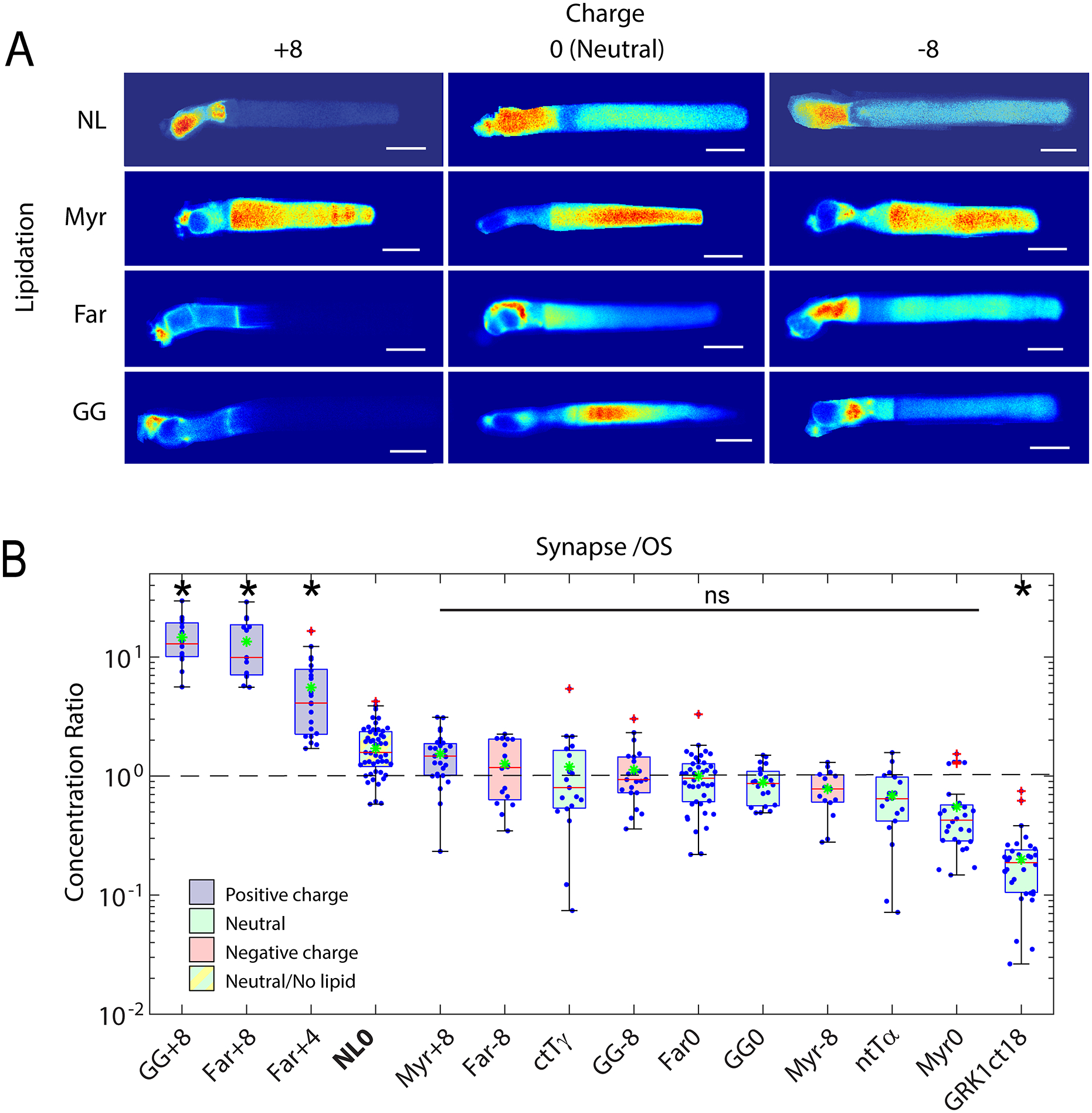
A. Montage of confocal images of living Xenopus rods expressing EGFP probes with indicated surface charge and lipidation motif. Note that none of the probes possess binding motifs for the lipid binding chaperone proteins, PrBPδ and Unc119. Significant OS localization of most probes shows that lipid binding chaperone proteins are not required for OS access and enrichment of peripheral membrane proteins. B. Box-whisker plots of average fluorescence in the pre-synapse divided by average fluorescence in the OS shows that positively charged probes with prenyl lipids are depleted from the OS and enriched in the pre-synapse, while probes containing myristoylation and neutral or negative charge equally distributed between compartments. A probe consisting of EGFP fused to the myristoylation motif containing N-terminal 16 amino acids of Tα, which binds to Unc119, was not significantly more OS enriched than the Myr0 probe, which does not bind Unc119, suggesting that Unc119 association alone is not sufficient for OS enrichment. The probe containing the farnesylated C-terminus of GRK1, which does bind to PrBPδ, is more strongly OS localized, thus, PrBP delta tilted the equilibrium toward OS enrichment. However, presence of the Far0 probe in the OS shows that PrBPδ is not required for OS entry. Modified from [130].
