Fig. 5. Steric volume exclusion and the compartmentalization of soluble proteins in photoreceptors.
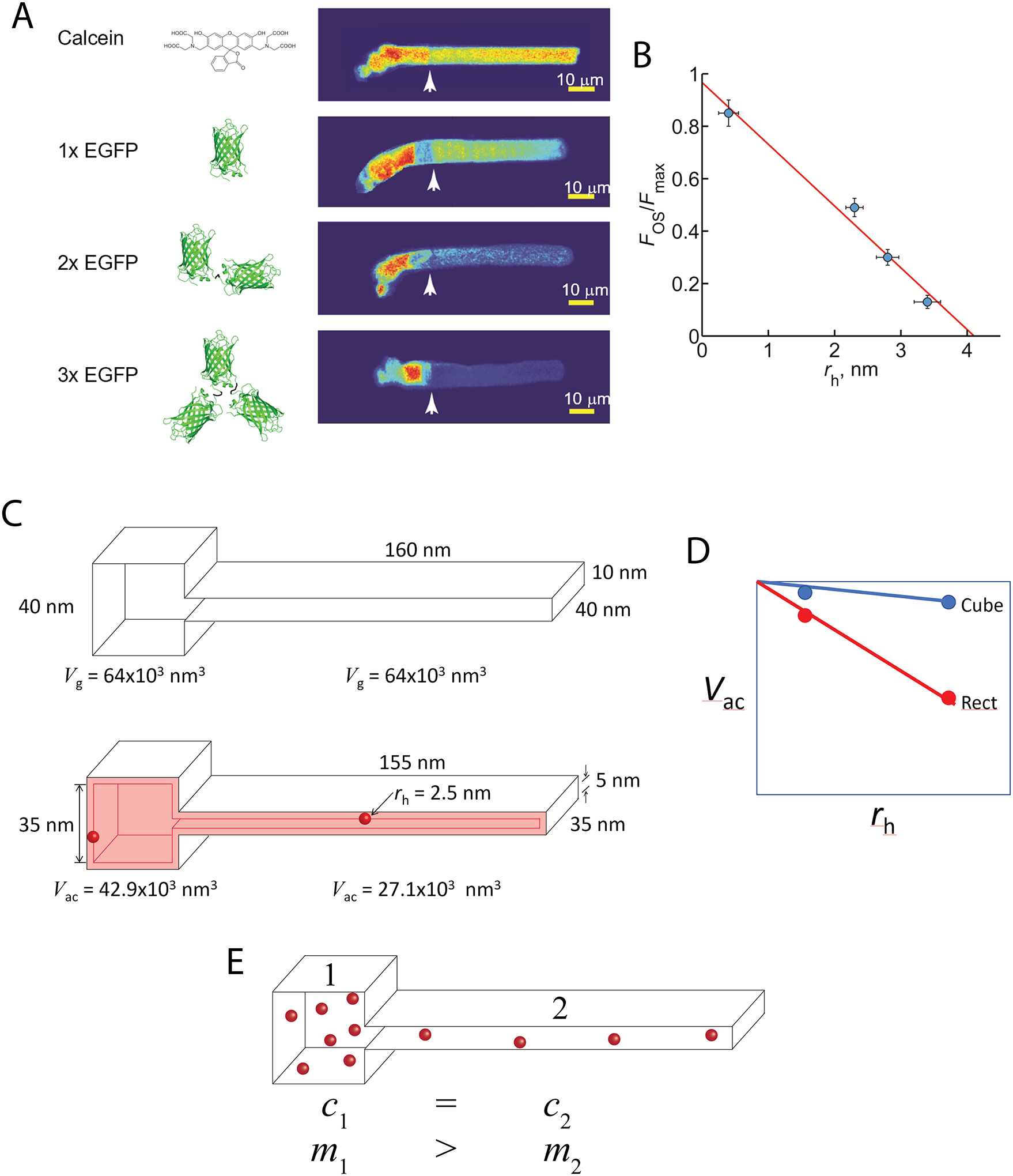
A. The distribution of soluble molecules in Xenopus rods depends on the size of the molecule. Note that the conformation of the EGFP dimers and tetramer shown are only one of many possible. B. The relationship of the ratio of the OS fluorescence to the maximum IS fluorescence scaled inversely and linearly with the estimated average hydrated radius of the molecules. This phenomenon can be explained by the asymmetrical reduction in the available aqueous volume of the differently shaped compartments caused by steric volume exclusion (i.e. loss of volume available to the center of mass of the molecule). C. For example, two interconnected boxes have the same geometric volume (Vg), but vastly different shapes. Introducing a spherical molecule reduces the geometry of both compartments, and thus the volumes accessible (Vac) to their centers of mass of the molecule. This reduction is larger for the rectangular compartment. D. As the size of the molecules increase, the reduction in Vac falls more steeply for the rectangular compartment. E. Since soluble molecules will equilibrate to equalize their concentrations (c) everywhere, the shape asymmetry will cause partitioning of the soluble molecules into the cubical compartment, where the total mass (m) will be higher. Panels A and B modified from [147].
