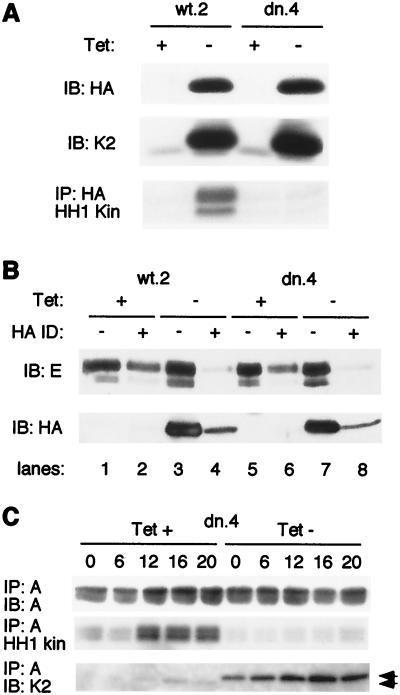
FIG. 7.
Induced Cdk2-wt and Cdk2-dn each associate with the majority of endogenous cyclin A and E, but Cdk2-dn is catalytically inactive. (A) Induced Cdk2-dn is catalytically inactive, whereas induced Cdk2-wt retains catalytic activity. Cells from clones wt.2 and dn.4 were incubated in the presence or absence of Tet for 24 h. Whole-cell extracts were subjected to immunoblotting (IB) with an antibody directed against the HA tag (top), immunoblotting with an antibody directed against Cdk2 (K2; middle), and immunoprecipitation (IP) with an antibody directed against the HA tag, followed by in vitro kinase assays using histone H1 (HH1 Kin) as a substrate (bottom). (B) Induced Cdk2-wt and Cdk2-dn each associate with the majority of endogenous cyclin E. Two successive rounds of immunodepletion (ID) with the HA antibody were performed on the extracts described above. Cyclin E (top) and induced Cdk2 (wt or dn, using the anti-HA antibody; bottom) levels in the extracts were determined by immunoblotting before and after immunodepletion. (C) Induction of Cdk2-dn abolishes most of the endogenous cyclin A-associated kinase activity. Dn.4 cells were synchronized at the G1/S border with HU. The HU was removed, and extracts were prepared from cells at the designated intervals. Cyclin A immunoprecipitates were subjected to immunoblotting with an anti-cyclin A antibody (top), examined for associated kinase activity, using histone H1 as a substrate (middle), or subjected to immunoblotting with an anti-Cdk2 antibody (bottom). Arrowheads, exogenous (upper band) and endogenous Cdk2.