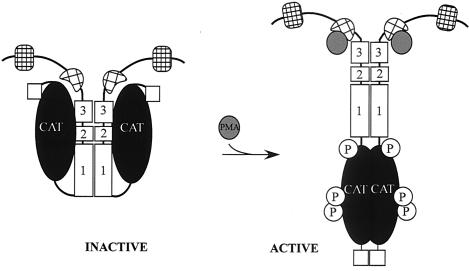
FIG. 10.
Model for regulation of the catalytic activity of MRCKα. The intramolecular interaction between the CC autoinhibitory domain CC2-CC3 and the kinase domain keeps the kinase in a closed, inactive, dimeric structure. Disruption of this interaction (e.g., PMA binding to the CRD or coexpression with a mutant kinase domain) resulted in an open structure that facilitates N terminus-mediated dimerization, autophosphorylation, and subsequent kinase activation.