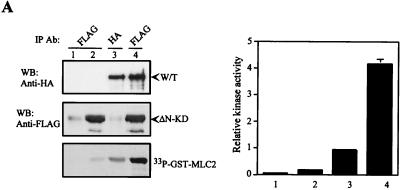
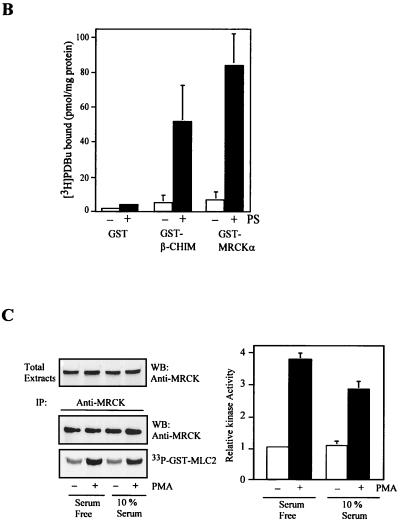
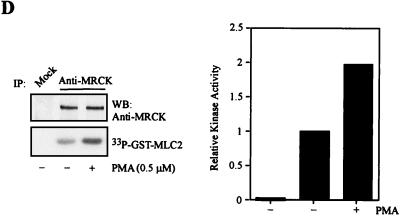
FIG. 9.
Activation of MRCK by interactive binding to distal CC and CRD. (A) COS-7 cells were transfected with either the pXJ40-HA-MRCKα (lane 1), the FLAG-MRCKα-ΔN-KDK106A,70–473 (lane 2), the HA-MRCKα (lane 3), or the HA-MRCKα plus the FLAG-MRCKα-ΔN-KDK106A,70–473 (lane 4) construct. Immunoprecipitations (IP) were carried out using either anti-HA or anti-FLAG antibody (Ab) as indicated. Immunoprecipitated products were subjected to kinase assay using GST-MLC2 (left). The phosphorylation levels quantified are expressed as means and standard errors of activities relative to that of HA-MRCKα (lane 3; right). W/T, wild type; WB, western blot. (B) Phorbol ester binding for MRCKα-CRD. GST fusion proteins were incubated with [3H]PDBu in the presence or absence of phosphatidylserine (PS) as described in Materials and Methods. The values shown are the means and standard deviations from three independent experiments. (C) Serum-starved and unstarved HeLa cells were treated either with 0.01% dimethylsulfoxide (control) or with PMA at 300 ng/ml in dimethylsulfoxide for 30 min. The endogenous MRCK was immunoprecipitated using an anti-MRCK antibody and subjected to kinase assays using GST-MLC2 as the substrate (left). Quantified GST-MLC2 phosphorylation levels are shown on the right as means and standard errors of activities relative to that of the MRCK immunoprecipitated from control serum-starved cells. (D) Concentrated extracts of serum-starved HeLa cells were preincubated with either 1% dimethylsulfoxide (control) or 0.5 μM PMA in the presence of 2 mM ATP and 2 μg of PS at 30°C for 20 min. The endogenous MRCK was then immunoprecipitated and subjected to kinase assays as previously described (left). Quantified GST-MLC2 phosphorylation levels are shown as means and standard errors of activities relative to that of the control from three independent experiments (right).