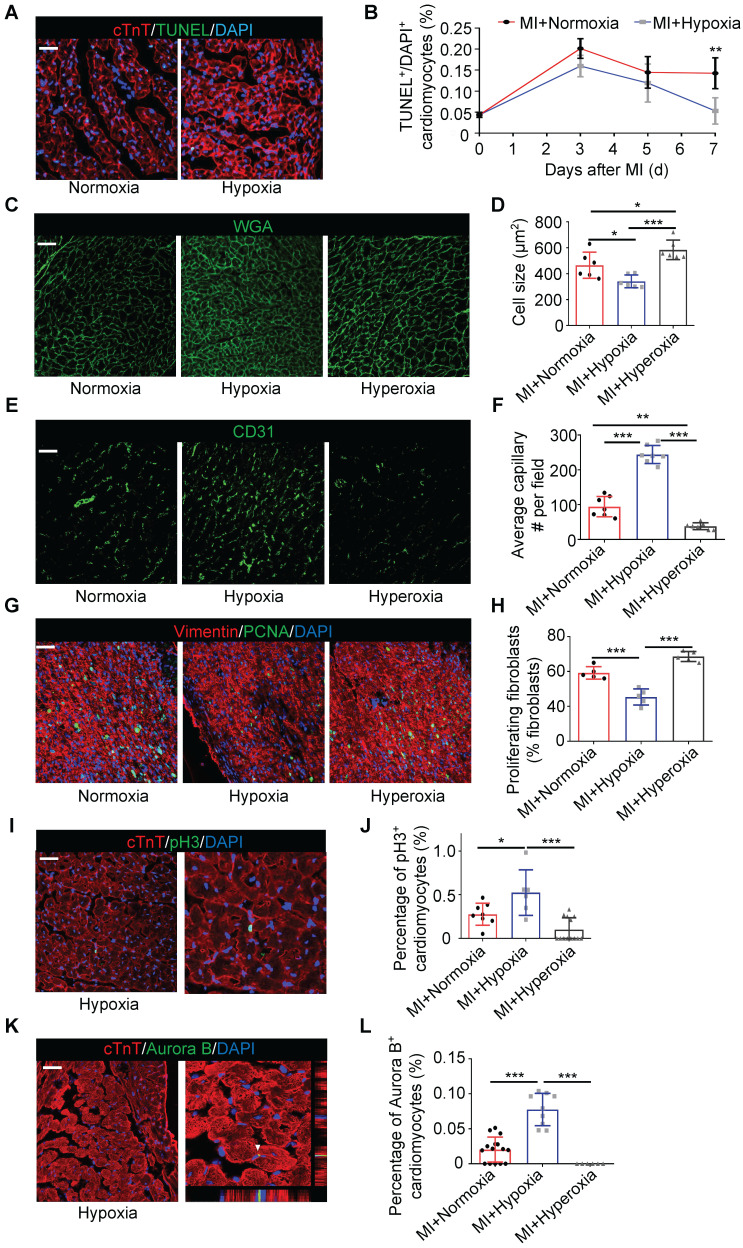
Figure 2.
Hypoxia mitigated cardiomyocyte apoptosis, induced angiogenesis and promoted cardiomyocyte proliferation. (A) Representative immunofluorescence images of TUNEL-stained heart tissue from the border zones of normoxia- and hypoxia-treated MI mouse hearts. (B) Quantitative assessment of cardiomyocytes in A and similar images revealed reduced TUNEL positivity in the hypoxia-treated MI mouse hearts at different time points (n = 3 at each time point). (C) Representative immunofluorescence images of wheat germ agglutinin (WGA)-stained heart tissue in the border zone. (D) Pooled analyses of the cardiomyocyte size. (E) Representative immunofluorescence images of CD31-stained heart tissue in the border zone. (F) Quantification of CD31 positivity surrounding cardiomyocytes. (G) Representative images of coimmunofluorescent staining of proliferating cardiac fibroblasts in the infarct area of mice under normoxia, hypoxia and hyperoxia 7 days postinfarction (MI). (H) Quantification of proliferating cardiac fibroblasts in the infarct area of mice. (I) Representative immunofluorescence images of coimmunostaining with anti-pH3 and anti-cTnT antibodies in the border zones of MI hearts. (J) Quantification of pH3+ cardiomyocytes after MI in the hypoxia and normoxia groups. (K) Representative immunofluorescence images of coimmunostaining with anti-Aurora B and anti-cTnT antibodies in the border zones of MI hearts. (L) Quantification of Aurora B+ cardiomyocytes after MI in the hypoxia and normoxia groups. Dot-plots represent data from individual mice. Statistical significance was determined by ANOVA followed by Sidak's multiple comparisons test. *P < 0.05, **P < 0.01 and ***P < 0.001 compared to the corresponding MI+normoxia. Scale bar: 50 μm.