Abstract
The consumption of linoleic acid (LA, ω−6 18:2), the most common ω−6 polyunsaturated fatty acid (PUFA) in the Modern Western diet (MWD), has significantly increased over the last century in tandem with unprecedented incidence of chronic metabolic diseases like obesity and type 2 diabetes mellitus (T2DM). Although an essential fatty acid for health, LA was a very rare fatty acid in the diet of humans during their evolution. While the intake of other dietary macronutrients (carbohydrates like fructose) has also risen, diets rich in ω−6 PUFAs have been promoted in an effort to reduce cardiovascular disease despite unclear evidence as to how increased dietary LA consumption could promote a proinflammatory state and affect glucose metabolism. Current evidence suggests that sex, genetics, environmental factors, and disease status can differentially modulate how LA influences insulin sensitivity and peripheral glucose uptake as well as insulin secretion and pancreatic beta-cell function. Therefore, the aim of this review will be to summarize recent additions to our knowledge to refine the unique physiological and pathophysiological roles of LA in the regulation of glucose homeostasis.
Keywords: Linoleic acid, Omega-6 PUFA, oxylipins, modern western diet, diet-heart hypothesis, type 2 diabetes mellitus
[1]. Introduction
The global prevalence of obesity and type 2 diabetes mellitus (T2DM) has tripled since 1975 [1, 2]. Although their underlying molecular pathophysiology is not yet fully understood, obesity and T2DM are often associated with diet-mediated disturbances in glucose and lipid metabolism [3, 4]. The Modern Western diet (MWD), which is commonly enriched in fat, salt, and refined sugar, has been implicated in the pathogenesis of these chronic metabolic “civilization” diseases [5–7]. Globalization and advances in agricultural technology throughout the 20th century have helped drive the constituents of the MWD, but advocacy efforts by influential health associations in the 1970s, which were founded on earlier landmark studies throughout the 1960s, have also led to unprecedented changes in the quantity and quality of fat content in the MWD [5, 7, 8–14]. In particular, the substitution of saturated fats for ω−6 PUFAs (mainly linoleic acid (LA, ω−6 18:2)), has been advocated by the American Heart Association (AHA) [8–10], United States Department of Agriculture (USDA) [12], American Diabetes Association (ADA) [13], and the National Institutes of Health (NIH) [14], largely based on evidence generated by the first comparative population-based study to relate diet and cardiovascular disease, The Seven Countries Study (SCS).
The SCS was initiated in 1958 by Ancel Keys and examined 12,763 males, 40–59 years of age, in multiple countries (seven were chosen for the final analysis) over 25 years in order to determine the connections of both lifestyle and anthropometric measures to the risk of developing or dying from cardiovascular disease (CVD) [15–18]. The study found that CVD-associated death rates were relatively low in countries where saturated fat comprised less than 10% of total calories consumed (Japan and Italy), but significantly greater in countries where saturated fat consumption was more than 30% of the diet (United States, Canada, and Australia) [15–18]. Considering these results, Keys and his colleagues later published contentious epidemiologic data concluding that diets rich in saturated fatty acids (SFAs) lead to an increase in blood cholesterol levels and CVD-associated mortality, while diets high in monounsaturated fats (MUFAs) and PUFAs (i.e., the Mediterranean diet) reduce CVD-related deaths [15–20].
Keys’ epidemiologic data provided a foundation for what eventually became known as the “diet-heart” hypothesis. The diet-heart hypothesis proposes that particular dietary components increase blood cholesterol levels and that an elevated blood cholesterol concentration is causally linked to an increased risk of coronary heart disease (CAD) [9]. In 1961, the Central Committee for the Medical and Community Program of the AHA formally identified SFAs as the “dietary component” responsible for CAD, citing “evidence from many countries suggests a relationship between the amount and type of fat consumed, amount of cholesterol in the blood, and the reported incidence of coronary artery disease” [9]. Justifying their decision with Keys’ studies among others [21–23], the AHA committee called for the replacement of dietary SFAs with common vegetable oils rich in ω−6 PUFAs (corn, cotton, and soya) as a means of preventing atherosclerosis and decreasing the risk of heart attacks and strokes [9].
In the years that followed, epidemiologic studies, interventional clinical trials, and meta-analyses aimed to validate the diet-heart hypothesis [24–33]. Nearly all of these studies found evidence that the replacement of SFAs with ω−6 PUFAs could lower serum cholesterol, yet data concerning the effect of the dietary substitution on clinical end points remain controversial [32–33]. In the United States, CVD-associated mortality has steadily decreased from 588.8 deaths per 100,000 people in 1950 to 163.6 deaths per 100,000 people in 2018 [34, 35], but the prevalence of diagnosed diabetes increased from 0.93% in 1958 to 12.00% in 2016 [34], and the percentage of Americans who are obese (BMI ≥ 30) has starkly risen from 13.3% in 1960 to 42.4% in 2018 [37]. The question remains as to whether the change in the dietary fat quantity or quality or a combination of both accounts for the increased prevalence of obesity and T2DM. As for increasing an individual’s dietary intake of certain species of PUFAs there is conflicting evidence on the effect that these dietary PUFAs have on the pathogenesis of obesity and T2DM [38–41].
Historically, the ratio of dietary ω−6 PUFA to ω−3 PUFA (ω−6: ω−3) has been used an indicator to measure risk associated with the development of some chronic metabolic disorders [42]. A greater dietary ω−6: ω−3 ratio has generally corresponded with the stimulation of inflammatory pathways that are prothrombotic and proaggregatory, while a lower ω−6: ω−3 ratio is thought to lead to the dominance of anti-inflammatory resolvins, protectins, and maresins [43]. Evolutionary studies have estimated that the n-6: n-3 ratio of the human diet was historically 1:1, but advocacy efforts throughout the 20th century by health agencies to increase ω−6 PUFA consumption [8–14] have resulted in an increase in the modern Western diet ω−6: ω−3 ratio to an average of 10: 1 [44].
Several reviews have proposed reducing the ω−6: ω−3 ratio as a primary preventive measure to protect against various cancers, CVD, TD2M, obesity, as well as other chronic metabolic disorders [45–49]. In general, the studies referenced by these reviews have provided promising data on a variety of benefits associated with lower ω−6: ω−3 ratio diets, but it is clear additional research is needed. For example, studies from India evaluating the effects of diets with different ω−6: ω−3 ratios on insulin action found that a lower ω−6: ω−3 ratio (6:1) could reduce the prevalence of T2DM [50]. Similarly, the Lyon Heart Study, which compared a Cretan Mediterranean diet (LA:ALA, 4:1) to the Step 1 AHA Diet (control, <30% of total energy derived from total fat, 7–10% of total energy from SFAs) found that lower ω−6: ω−3 ratio diets led to a 70% decrease in total mortality [51]. While these RCTs provide some evidence that lowering the dietary ω−6: ω−3 ratio could be an effective preventative measure against the development of metabolic disorders or premature death, few studies have examined an upper limit of intake of dietary ω−3 PUFAs. Studies that have reported the overconsumption of ω−3 PUFAs indicate both potential deleterious and beneficial effects such as increased serum glucose [52], significantly decrease coagulation [53], and reduce blood pressure [54]. Taken together, it is possible the consumption of specific dietary PUFA species may indeed be governed by the Goldilocks Principle, whereby moderation of ω−6 PUFA and ω−3 PUFA intake produces optimal metabolic effects.
Linoleic acid (LA; ω−6, 18:2), an essential fatty acid, has become the most abundant PUFA in the MWD. Since 1960, the average intake has steadily risen from 2.7 g/day to approximately 4.9 g/day to 21.0 g/day (~4–10% of total dietary calories) in Western countries [42, 55–58]. Over the last century, changes in the consumption of other dietary fatty acids such as arachidonic acid (AA; ω−6, 20:4), eicosapentaenoic acid, docosapentaenoic acid, and docosahexaenoic acid have been unremarkable, but some like α-linoleic acid have risen (from 0.39% to 0.72% of energy). Regardless, increases in LA intake have been the most substantial compared to other dietary fatty acids [42]. Although LA is an essential fatty acid to form our skin vapor barrier [59], LA was an exceptionally rare fatty acid in the diet during human evolution as evidenced by a human’s ability to dramatically concentrate low levels of dietary LA [61]. While a daily intake of LA around 1 to 2% of total dietary calories is sufficient to prevent the development of essential fatty acid deficiency [59, 60], the over 10-fold increase in LA consumption since the mid-20th century has prompted further research as to how LA could impact long-term health through its metabolic effects on pancreatic beta-cell function and peripheral glucose uptake. Thus, the aim of this review will be to summarize recent findings that characterize the unique physiological and pathophysiological roles of LA in the regulation of glucose homeostasis.
[2]. Molecular Evidence for the Role of Linoleic Acid in Glucose Metabolism
2.1. Normal Glucose Homeostasis
Under normal fasting conditions (10–12 hours post-meal), circulating glucose levels are kept constant through the balance of endogenous glucose production in the liver and kidney with systemic glucose uptake and utilization (glucose disposal) [62]. Glucagon partially facilitates glucose production, while insulin mediates glucose uptake in insulin-sensitive tissue [62–64]. In the former, glucagon plays a key role in sustaining plasma glucose by first stimulating hepatic glycogenolysis and then later gluconeogenesis during prolonged periods of fasting [64]. Glucose disposal, in contrast, is partitioned such that the majority (~75%) of whole-body glucose is taken up by insulin-independent tissues like the brain, liver, and gastrointestinal tract, and the remaining glucose (~25%) is disposed of by insulin-dependent peripheral tissues (mostly skeletal muscle) [62, 63]. An increase in plasma glucose during the postprandial state stimulates pancreatic beta-cells to secrete insulin, which reduces circulating glucose by antagonizing glucagon secretion and promoting the majority of insulin-mediated glucose uptake to occur in the skeletal muscle (80–90%) [64–66]. While there are several other glucoregulatory hormones involved in this equilibrium, insulin, in particular, plays a critical role in the maintenance of normal physiologic blood glucose levels in the fasting and fed states.
Different macronutrients (i.e., fatty acids, amino acids, and sugars) can stimulate beta-cells to secrete insulin, but glucose serves as the principal secretagogue [67]. As such, the current model for glucose-stimulated insulin secretion (GSIS) is that extracellular glucose is transported through GLUT1 or GLUT2 where intracellularly it is irreversibly phosphorylated by glucokinase in a rate-limiting, ATP-dependent reaction. Glucokinase is a specialized hexokinase because of its higher Km for glucose than others in the hexokinase family and it is expressed only in the liver and beta-cell [68]. With the higher Km for glucose, glucokinase functions as the primary glucose sensor in mammals and regulates the rate of glucose entry into the glycolytic pathway [69, 70]. Glucose-6-phosphate produced by glucokinase in the beta-cell is metabolized by glycolysis to yield ATP, which increases the cytoplasmic ATP/ADP ratio and leads to depolarization of the plasma membrane, causing influx of extracellular calcium, and exocytosis of insulin granules [67, 70]. Through the complex interplay of these molecular processes alongside others like filamentous actin (F-actin) cytoskeletal remodeling, GSIS functions as a critical regulatory mechanism in the balance of whole-body glucose levels.
Certain non-esterified fatty acids (NEFAs) are capable of stimulating insulin secretion and changes in plasma NEFA levels have been shown to directly affect pancreatic beta-cell function. In classic experiments, McGarry et. al was the first to demonstrate circulating NEFAs are essential for basal insulin secretion and the maintenance of normal GSIS [70]. These findings have been confirmed in vitro and in vivo, whereby reports have determined an acute increase in plasma NEFAs (<48 hours, 500–800 μmol/L) either enhances [71–73] or has no effect on GSIS [74, 75]. Paradoxically, chronically elevated plasma NEFA levels (>48 hours, 500–800 μmol/L) impair insulin secretion, although this effect is dependent on the composition of NEFAs in the blood [62, 63, 76, 77]. Unfortunately, the underlying molecular mechanisms by which acute increases in NEFA levels enhance GSIS or chronically elevated NEFA levels impair GSIS are not completely understood.
While plasma NEFAs appear to augment or impair insulin release in a temporal manner, the physiological responses are differentially affected by the type of NEFA species in terms of chain-length and degree of unsaturation. For example, acute exposure (40-minutes), McGarry et al. showed that the insulinotropic potency of NEFAs increases with their degree of saturation, where stearate (SA, 18:0) and palmitate (PA, 16:0) nearly doubled insulin output from perfused rat pancreata when compared to oleate (OA, 18:1) and linoleate (LA, 18:2) [71]. Further, long-chain NEFA species like PA could stimulate nearly four times as much insulin secretion than short-chain NEFA species like octanoate (OcA, 8:0). Thus, the insulinotropic potency of NEFAs is greatest for long chain SFAs, followed by long chain FAs with increasing desaturation (SFAs > MUFAs > PUFAs), and then short chain FAs (C8 or less).
Later experiments examining the effects of short-term NEFA exposure on GSIS agree with McGarry et al. 1999 [72, 73], but others show that the long-term insulinotropic potency of dietary FAs is also species-dependent [72–74]. Using rats fed either a low-fat, lard diet (SFA/MUFA), or soybean oil (SO) diet (53% LA) for 4-weeks, Dobbins et. al found that insulin secretion by hyperglycemic clamp was enhanced in the lard-fed rats and impaired in the SO-fed rats [77]. The negative effects of soybean oil diet on insulin secretion have also been observed in diabetic (Goto-Kakizaki) and normal Wistar rats, where only 7-days of SO diet feeding impaired GSIS in both groups [78]. Similarly, in vivo human studies show that obese males ingesting SO shakes over 24-hrs had impaired GSIS during hyperglycemic clamp [79], but these aforementioned studies should be considered with a disclaimer. Historical references to dietary components such as lard or canola oil inaccurately reflected the fatty acid composition of dietary oils, but now this is no longer the case. With commercial analytical services being so inexpensive, the authors hope that the field moves towards defining the exact fatty acid composition of nutritional components in nutritional experiments as well as the level of oxidized species and other impurities. Regardless, current evidence indicates the duration of exposure and specific composition of dietary FAs, including chain-length, degree of unsaturation, and concentration, can either potentiate or diminish insulin secretion.
Besides their role in GSIS, small amounts of dietary FAs and many of their downstream metabolites like oxylipins have species-dependent properties that can yield auxiliary effects on the regulation of membrane structure and fluidity, chemical mediation, intracellular signaling pathways, gene expression, immune signaling and activation, as well as inflammatory responses that may further modulate glucose homeostasis [79]. Additional research will be needed to clarify the complex biochemical relationships between dietary FAs and whole-body glucose homeostasis, but it is clear that dietary FAs can positively or negatively impact glucose metabolism in both normal and disease states. Thus, it is critical to determine if the increased consumption of certain FAs in the diet, like LA as is currently advocated in nutritional guidelines endorsed by health agencies like the AHA (2017 Presidential Advisory) [8], USDA (Dietary Guidelines for Americans, 2020–2025) [12], and ADA (Lifestyle Management: Standards of Medical Care in Diabetes-2019) [13], have potential adverse or unintended physiological consequences.
2.2. Metabolism of Linoleic Acid, Bioactive Oxidized Linoleic Acid Metabolites, and Eicosanoids
In human cells, unesterified intracellular LA can be 1) activated by the rate-limiting ATP-dependent long-chain acyl-CoA synthetase (ACSL)-mediated thioesterification with coenzyme A (CoA) [80–82], 2) converted to oxidized LA metabolites (OXLAMs) by lipoxygenases (5-, or 12/15-LOX), cytochrome P450s (CYP-2C, 2J, 2E, 4A, or 4F), and epoxide hydrolases [83–85], or 3) converted to AA and other ω−6 PUFAs by a series of desaturases and elongases [82] (Figure 1). Linoleoyl-CoAs generated by ACSL activity are generally channeled towards β- and ω-oxidation or esterified into complex lipids depending on cellular energy demands and environmental cues [82]. OXLAMs produced by oxylipin-producing enzymes can play a role in inflammation, pain perception, gene regulation, and oxidative stress responses [86]. Finally, LA-derived AA can contribute to intracellular eicosanoid production, which mediates cellular signaling in multiple pathological and physiological processes [87, 88].
Figure 1: Metabolic Fates of Intracellular Non-esterified Linoleic Acid.
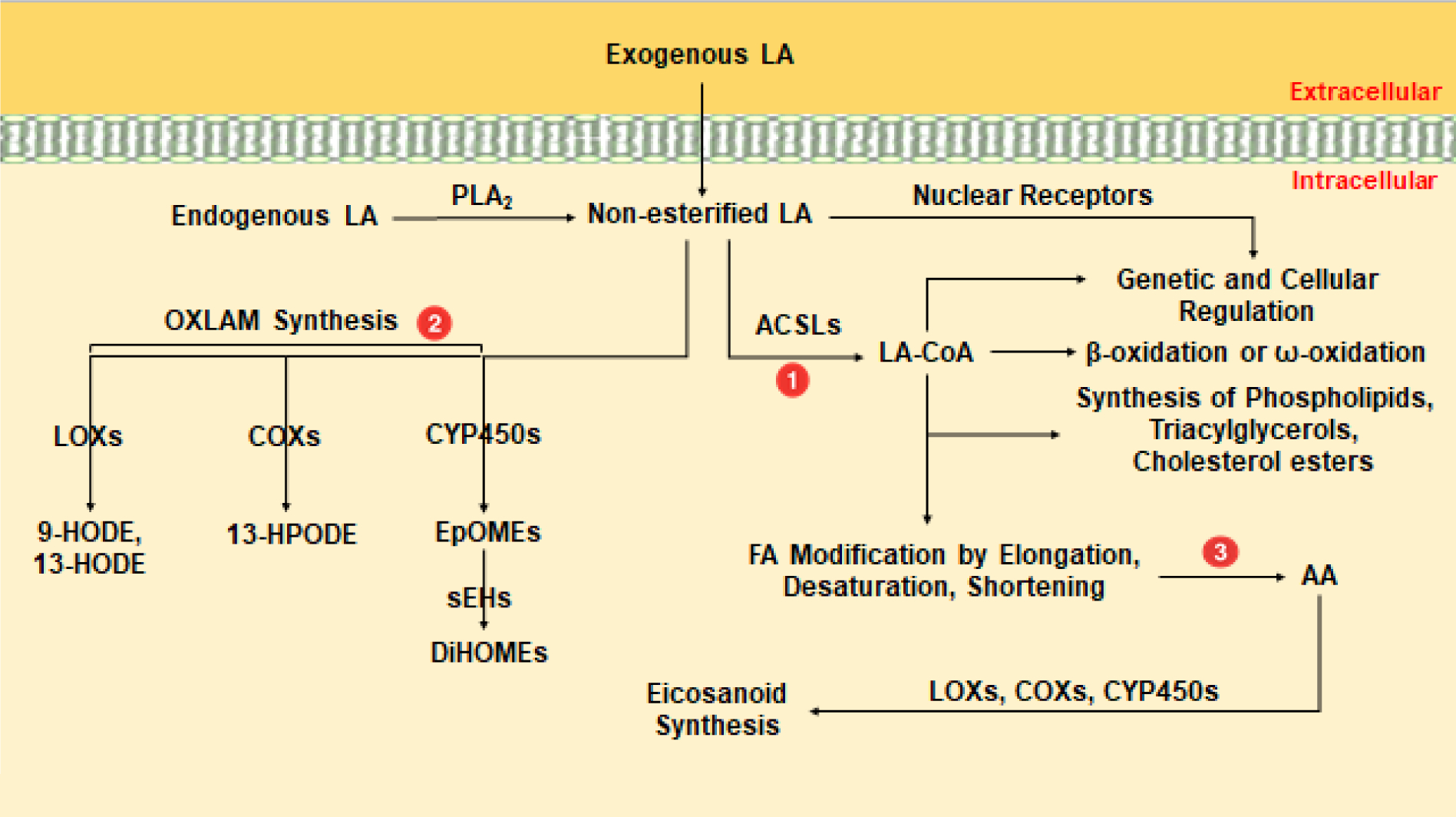
Intracellular metabolism of non-esterified linoleic acid (LA). Abbreviations: phospholipase A2 (PLA2), acyl-CoA synthetase long-chain (ACSL), linoleoyl-CoA (LA-CoA), oxidized linoleic acid metabolites (OXLAM), lipoxygenase (LOX), cyclooxygenase (COX), cytochrome P450 (CYP450), 9-hydroxyoctadecadienoic acid (9-HODE), 13-hydroxyoctadecadienoic acid (13-HODE), 13-hydroperoxyoctadecadienoic acid (HPODE), epoxyoctadecamonoenoic acids (EpOMEs), soluble epoxide hydrolase (sEHs), dihydroxyoctadecenoic acids (DiHOMEs), arachidonic acid (AA).
In the initial event of LA metabolism, all 5 ACSL isoforms (ACSL1, −3, −4, −5, −6) have the potential to catalyze the synthesis of linoleoyl-CoA, although in vitro evidence suggests ACSL6 has a greater substrate preference for LA than the other ACSLs [80]. As primarily membrane bound proteins, ACSL isoform cellular location and substrate preference provide an indication as to how and where their FA substrates will be channeled towards or away from specific pathways [82]. ACSL isoforms are ubiquitously expressed, but ACSL enzymes differ in their tissue and cellular expression. For example, ACSL1 is predominantly found in the liver, ACSL3 in the testis, ACSL4 in the brain, adrenal glands and other steroid producing organs, ACSL5 in the small intestine, and ACSL6 in both the brain and gonads [81]. At the cellular level, ACSL1, −5, and −6 are primarily associated with the mitochondria, plasma membrane, and cytoplasm, while ACSL3 is found in the endoplasmic reticulum (ER) and ACSL4 in both peroxisomes and ER [80, 81]. Among its many fates in this pathway, linoleoyl-CoA can be esterified into complex lipids (phospholipids, triglycerides, and cholesterol esters), degraded in peroxisomes, mitochondria, and the ER for energy production, or used to regulate gene expression and other cellular processes [82].
Like LA-CoA metabolites, bioactive OXLAMs can be channeled towards multiple metabolic fates and their concentrations in the blood are directly proportional to dietary LA consumption [83]. The first class of bioactive OXLAMs consists of the LOX-derived 9- and 13 hydroxy-octadecadienoic acid (9- and 13-HODE) and 9- and 13-oxo-octadecadienoic acid (9- and 13-oxoODE), which appear to play a role in inflammatory responses and have been mechanistically linked to CVD, non-alcoholic steatohepatitis (NASH), elevated LDL levels, and chronic pain [83]. A recent study also implicated these species as potent antagonists of insulin signaling through the FOXO family of transcription factors [84]. Besides HODEs, epoxyoctadecamonoenoic acids (EpOMEs) and their downstream diols, dihydroxyoctadecenoic acids (DiHOMEs), are another clinically significant family of bioactive OXLAMs [85, 86, 91]. In the former, CYP enzymes, which are selective, but by no means specific in producing epoxides over other oxylipin metabolites, convert LA to the LA epoxides 9,10-epoxyoctadecenoic acid (9,10-EpOME) and 12,13-epoxyoctadecenoic acid (12,13-EpOME) [91] These epoxides are known as leukotoxin and isoleukotoxin respectively [90–92]. EpOMEs can participate in the induction of inflammation and immune reactions, but as previously mentioned can also be rapidly metabolized into DiHOMEs by soluble epoxide hydrolase (sEH) and microsomal epoxide hydrolase (mEH) [93]. Epoxide hydrolases vary dramatically with species and tissue, but ultimately sEH appears to be the major hydrolytic enzyme involved in the production of DiHOMEs from EpOMEs, while mEH plays a more significant role in the epoxidation of FAs in only certain tissues [93].
DiHOMEs have been studied for their involvement in neutrophil respiratory bursts, chemotaxis, tissue injury, as well as pain in both human and animal models [85, 86, 89–91]. Despite their cytotoxic properties when present in the blood at high concentrations (>100 μM) for extended periods of time (>2 hours), DiHOMEs have species-specific effects on the body, some of which could have important regulatory functions in glucose homeostasis [90, 91]. For example, in vivo experiments have shown that 12-,13-DiHOME increases NEFA uptake into brown adipose tissue (BAT), and that its plasma concentration negatively correlates with serum glucose in humans, while positively correlating with improved peripheral glucose uptake in mice [94]. On the contrary, recent studies studying adipogenesis and thermogenesis have referred to oxylipins of LA with terms such as adipokines, lipokines, and thermokines [91], Thus it may not be clear from this nomenclature that the aforementioned chemokines described in the literature are actually produced from LA. It is apparent that further research is needed to better understand the complex biological roles that OXLAMs may play in glucose homeostasis.
Lastly, a small amount of unesterified LA (0.2–10%) can be converted to AA through the action of desaturases (delta-6, delta-5) and elongase-5 [95]. This poor rate of conversion may be result of LA partitioning into other pathways, but competition of LA with other ω−3 PUFAs (α-linoleic acid, stearidonic acid, and eicosapentaenoic acid) may be also responsible, as they serve as substrates for a shared supply of intracellular elongases and desaturases. While relatively little LA is metabolized to AA, several studies have found that diets rich in LA can still significantly raise plasma AA-derived bioactive lipids [96–98], which is important as AA serves as the parent molecule for all inflammatory eicosanoids [99]. Once AA is converted to prostaglandins, leukotrienes, and epoxides by COX, LOX, and CYP respectively, the AA-derived metabolites function as potent signaling molecules by regulating vasodilation and vasoconstriction, ion channel activation, angiogenesis, mitogenesis, inflammatory responses, and hormone secretion across multiple cell types [99]. Thus, through this final pathway, the biosynthesis of AA potentially links dietary LA intake to the wide array of downstream metabolic consequences associated with the generation of eicosanoids.
Like other oxylipins, eicosanoids have a wide range of physiological tissue-specific effects that can influence glucose homeostasis. For example, CYP-derived AA metabolites like epoxyeicosatrienoic acids (EETs) have been shown to impair beta-cell insulin secretion [100]. Although it is not yet entirely clear how this occurs, we have previously shown that AA or LA exposure in INS 832/13 (rat) insulinoma beta-cells specifically reduces ACSL4 expression and impairs GSIS by as much as 30% through the resulting accumulation of unesterified EETs. Further, recent studies have also found that prostaglandins like PGE2 can promote peripheral insulin resistance through multiple molecular mechanisms in different tissues, including adipocytes via the PGE2-EP4 axis [101] and skeletal muscle through the overstimulation of sympathetic nerves by the ventromedial hypothalamus [102, 103]. While many other eicosanoids affect glucose metabolism, EETs and prostaglandins are becoming potential indicators to measure the effects of AA on the pathogenesis of diseases like T2DM and obesity.
In sum, the molecular actions of both OXLAMs and LA-derived AA metabolites provide considerable in vitro and in vivo evidence that dietary LA can potently affect glucose homeostasis. Coincidently, the concurrence of increasing trends in the consumption of LA and the worsening epidemic of chronic metabolic disease is not circumstantial, but rather linked to direct changes in the diet that have emerged as a result of nutrition advocacy efforts since the 1960s. Epidemiologic studies, meta-analyses, and clinical trials [15–33] have outlined the potential unintended consequences of diets substituting SFAs with ω−6 PUFAs, like LA, but the role that LA plays in either maintaining or disrupting glucose metabolism is still unclear. The discordance between findings from in vitro and rodent models and those of human epidemiologic studies has created confusion as to the potential beneficial or deleterious effects of LA on glucose homeostasis. While rodents have been extremely valuable to the molecular and mechanistic research these models do not necessarily translate to complex heterogenous human populations. Thus, one must use caution when applying findings and making dietary recommendations from any study as it pertains to the quantitative dietary patterns that drive modern human metabolic disease. Next, through the lens of T2DM, we review recent clinical evidence specifically focusing on the effects of dietary LA on peripheral glucose uptake, insulin resistance, and beta-cell function.
[3]. Clinical Evidence for the Role of Linoleic Acid in Glucose Metabolism
3.1. Peripheral Glucose Uptake and Insulin Resistance
Elevated fasting blood glucose (≥ 126 mg/dL after 8 hours of no caloric intake or 2-hour glucose ≥ 200 mg/dL during an oral glucose tolerance test) and glycated hemoglobin (A1C ≥ 6.5%) levels are diagnostic of diabetes mellitus [104]. Similar to the aforementioned studies examining the molecular effects of LA-derived metabolites, clinical trials have not reached a consensus as to how dietary LA affects blood glucose levels in either the fasting or fed state. Recently, a cross-sectional analysis of the Hoorn Study (n=667) found that unesterified serum LA was inversely associated with fasting glucose 2 hours after a 75-gram oral glucose challenge, but not with HbA1C in both non-diabetic and diabetic subjects [105]. However, when individuals with T2DM were excluded from the prospective analysis (n = 257), circulating LA was not associated with fasting or post-load glucose levels [105]. These differential associations suggest that dietary LA may exert a protective effect on fasting and postprandial glucose levels in individuals with T2DM, but not in subjects without diabetes.
To further complicate the relationship between dietary LA and blood glucose levels, a cross-sectional, population-based analysis of an all-male cohort diagnosed with metabolic syndrome (n = 1337) found that the effects of dietary LA on blood glucose levels are dependent upon which variant of the fatty acid desaturase-1 gene (FADS1) is expressed [106]. FADS1 encodes FADS1, an enzyme that is critical for the conversion of intracellular LA to AA, as it catalyzes the final desaturation of both ω−3 and ω−6 PUFAs at the delta-5 position to generate EPA and AA respectively [107]. In their analysis, Lankinen et al. found that individuals with either the rs174550-TT (n = 444) or rs174550-TC (n= 648) genotypes (less desaturase activity) had lower fasting glucose levels on average than those with a rs174550-CC (n= 245) genotype (more desaturase activity) in response to dietary intervention with LA (P < 0.001) [106]. While this study is the first of its kind to determine that the relationship between blood glucose levels and dietary LA can be affected by a common intron variant in a desaturase, current evidence suggests that the role of dietary LA in mediating blood glucose levels is complex and is likely modulated by multiple factors like diabetes status, genetics, and other environmental factors.
Another defining feature of T2DM is insulin resistance in both the liver and skeletal muscle [62, 63]. While the complete molecular mechanisms behind the development of hepatic and peripheral insulin resistance are not completely understood, current evidence suggests that insulin resistance is, in part, the result of complex insulin-induced transcriptional changes in the liver, skeletal muscle and adipose tissue (i.e., FOXO, CREB, and PGC-1α) that alter the expression of insulin receptors as well as key enzymes involved in gluconeogenesis like phosphoenolpyruvate carboxykinase 1 (PCK1) and glucose-6-phosphatase (G6PC) [108–110]. In vitro and in vivo experiments using rats have shown that induced hyperinsulinemia by long-term administration (>2 weeks) of insulin can decrease the number of insulin receptors on target tissues and thus diminish cellular insulin sensitivity [111, 112]. These reports have also been confirmed in humans, whereby Rizza et al. found that prolonged hyperinsulinemia (40 hours) reduced glucose utilization and glucose metabolism at submaximal and maximally effective plasma insulin concentrations [113]. Taken together, studies in rodents and humans indicate that hyperinsulinemia serves as the primary driver of insulin resistance [110–114].
Numerous studies have aimed to determine the role of LA in hyperinsulinemia and peripheral glucose uptake, yet the current clinical evidence is often divergent. In terms of the beneficial effects of LA, recent surveys of epidemiologic studies have found that higher levels of LA in the diet (determined by assessing feeding interventions) or blood (plasma or serum levels, erythrocyte phospholipids, and cholesterol esters) are associated with a lower risk of developing T2DM and improved insulin sensitivity [115–119]. In a systematic review and meta-analysis of the Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) Fatty Acids and Outcomes Research Consortium (FORCE), a pooled analysis of 39,740 non-diabetic individuals from 20 prospective cohort studies determined that all measured biomarkers of LA compartmentalization (phospholipids, total and serum plasma, cholesterol esters, and adipose tissue composition) were associated with a lower risk of developing T2DM (risk ratio per interquintile range 0.65, 95% CI 0.60–0.72, p<0.0001) [115]. Further, these associations of measured biomarkers of LA compartmentalization with T2DM were not significantly changed by age, BMI, sex, race, aspirin use, ω−3 PUFA levels in the blood, or variants of the FADS gene.
The findings of the CHARGE/FORCE studies on the benefits of LA intake are supported by other systematic reviews and meta-analyses [116, 117]. In the European Prospective Investigation into Cancer and Nutrition (EPIC)-Interact study [116], the analysis of 12,132 incident T2DM cases and 15,919 subcohort participants from eight European countries found that plasma LA was inversely associated with the risk of developing T2DM (0.80; 95% CI 0.77–0.83). Similarly, a recent systematic review and dose-response meta-analysis of prospective cohort studies found that for every 5% increase in energy from dietary LA intake, the risk of developing T2DM decreases by 10% [117]. However, the results from 9 cohorts (22,639 cases of T2DM) were largely driven by the findings of the Health Professionals Follow-Up Study [118,119] (exclusion from the analysis led to a loss of significance), the authors’ examination of LA tissue levels in 27 cohorts (18,458 cases of T2DM) provided clearer evidence that increased LA compartmentalization is protective against the pathogenesis of T2DM [117]. This meta-analysis suggests that there are unlikely to be harmful effects from increasing dietary LA. However, given inconsistent findings from dietary LA intake and difficulties in interpreting LA biomarker studies (LA compartmentalization), conclusions from these epidemiologic analyses regarding a role of increasing LA in the diet for the prevention of T2DM need to be made with caution.
Besides the inverse relationship between dietary LA intake and the incidence of T2DM, some studies have also measured the effects of dietary PUFA on insulin sensitivity. Using 102 RCTs with data on 4660 total participants, Imamura et al. determined that replacement of 5% dietary energy from carbohydrates with 5% energy with PUFA reduced fasting insulin by 1.6 pmol (0.4, 2.8; p = 0.015) [120], although these investigators did not specify which PUFA species were measured in these studies and assumed that “90%+ of total PUFA” was LA. Fasting hyperinsulinemia is frequently used as a surrogate measure of insulin resistance and has been shown to be predictive of developing T2DM in multiple populations [121–124]. Although this review was able to show increased dietary PUFA could decrease insulin resistance, the study was substantially limited by its inability to implicate a specific PUFA species to this effect and as such, could have overestimated the benefits of the substituting dietary carbohydrates with LA.
The preceding reports provide evidence in favor of a beneficial role of LA in glucose homeostasis, but numerous studies have also reported that LA contributes to insulin resistance and decreased peripheral glucose uptake in animal models and humans [125–129]. A recent study by Deol et al. compared the effects of four isocaloric diets (high-fat [40% total kcal] coconut oil diet rich in SFAs [36% kcal, 4% SO], high-fat [40% total kcal] SO diet rich in LA [19% kcal SO, 21% kcal coconut oil], high-fat [40% total kcal] coconut oil diet with fructose [25.9% kcal fructose, 36% kcal coconut oil, 4% SO], and high-fat [40% total kcal] SO diet with fructose [25.9% kcal fructose, 19% kcal SO, 21% kcal coconut oil]) on the pathogenesis of T2DM and obesity in C57/BL6 mice [125]. After 20 weeks, the investigators examined glucose tolerance and insulin sensitivity by a glucose tolerance test (GTT) and insulin tolerance test (ITT), respectively and found that mice on the high-fat diet with fructose did not have impaired glycemia (fasting blood glucose level > 200 mg/dL), while mice fed the high-fat SO diet were hyperglycemic. Additionally, mice on the high-fat coconut oil diet with fructose were less tolerant to glucose than the controls on a standard low fat, high fiber chow diet and mice fed the high-fat SO diet were glucose intolerant and insulin resistant [125]. The authors concluded that SO, which is 55% LA (18:2) [126], is more obesogenic and diabetogenic than fructose or coconut oil in mice [127]).
These results have been confirmed in other mouse studies, where investigators determined that diets enriched in SO led to excessive weight gain, higher levels of fasting blood glucose and insulin, increased levels of tumor necrosis factor-α, interleukin 6, high-sensitivity C-reactive protein, and reduced mRNA expression of cytoplasmic proteins that transmit signals from the insulin and insulin-like growth factor-1 receptors to produce a cellular response (insulin receptor substrate 1/2) [127]. Similarly, reports in other species have found that LA-enriched SO diets contribute to insulin resistance by disrupting the PI3K/AKT pathway in large yellow croaker fish [128] or by simultaneously increasing PPARγ gene expression alongside the suppression of adiponectin expression in the adipocytes of broiler chickens [129]. When all considered, these studies underscore the complexity of insulin resistance and demonstrate that multiple molecular mechanisms may contribute to reduced cellular insulin sensitivity in peripheral tissues.
In humans, several studies have examined LA-enriched (SO) diets for their effects on insulin resistance [130–132]. Nowotny et al. found that oral administration of a single dose of soybean oil (100 mL) could reduce whole-body insulin sensitivity (measured by hyperinsulinemic-euglycemic clamp) in the same timeframe and to a similar extent as an energy and composition-matched IV lipid-heparin infusion [130]. Other studies, like Naughton et al. and Kim et al., focus on the effects of LA on biomarkers of insulin resistance. For instance, Naughton et al. reported that LA-enriched diets lead to increased levels of resistin, an adipokine that decreases insulin sensitivity in obese individuals [131], while Kim et al. found that LA-enriched diets in healthy, non-diabetic subjects increase apolipoprotein B [132], another biomarker of insulin resistance [133]. Finally, some studies, like Zulkiply et al., found that in young to middle-aged, non-diabetic participants (n=333) LA-enriched diets have no significant associations with fasting insulin [1.72pmol/L (−11.39,14.84) p = 0.80] [134].
The combination of conflicting reports illustrates the need for additional large and rigorously-conducted RCTs like the Minnesota Coronary Experiment [135] and Sydney Diet Heart Study [136] in order to specifically evaluate the effects of dietary LA on the pathogenesis of T2DM. As historical meta-analyses and epidemiologic studies were frequently poorly controlled or based upon unreliable methodology (i.e. food diaries), well-controlled interventional RCTs could better inform current nutritional guidelines and clarify how dietary LA and other individual ω−6 PUFAs affect insulin resistance and peripheral glucose uptake in individuals with and without T2DM.
3.2. Beta-Cell Function and Insulin Secretion
Beta-cell viability and the molecular mechanisms that regulate insulin secretion are compromised in individuals with T2DM [137]. Although multiple studies have sought to evaluate the relationship between dietary LA and insulin secretion [138–142], a consensus has not yet been made as to how LA affects this critical arm of glucose homeostasis. Like other regulatory mechanisms in glucose metabolism, GSIS can be affected by sex [143], genetic variants [144], and environmental factors [145], however, no studies to date have been able to evaluate the role of LA in GSIS and account for these variables. Regardless, several reports do agree that acute (<24 hours) and chronic (≥24 hours) LA exposure differentially affect GSIS. Acute LA exposure (up to 90 minutes), although not as insulinotropic as other dietary FAs like PA or OA, can still augment GSIS as insulin secretion is more than double in human, rat, and mouse infused islets [139, 140, 146]. The underlying molecular mechanism is not completely understood, but the current hypothesis is that short-term LA exposure enhances GSIS by activating the G-protein coupled receptor, GPR40, that increases intracellular calcium in the beta-cell resulting in augmented insulin secretion [147, 148]. In contrast, chronic LA exposure impairs insulin secretion [100, 141, 144] and is thought to be a result of the accumulation of intracellular unesterified LA-derived metabolites [100, 149].
While the effects of increased unesterified LA-derived metabolites in beta-cells are not well understood, studies have argued that such changes could either enhance or impair insulin secretion [100, 149–151]. In terms of improving insulin secretion, Yaney et al. 2003 proposed that exposure to NEFAs like LA could increase intracellular concentrations of LA-CoAs and other complex lipids like diacylglycerols in beta-cells that would affect enzymes like protein kinase C or the exocytotic machinery [150]. Conversely, other studies have found that prolonged LA exposure increases the concentration of intracellular oxylipins that impair insulin secretion [100, 149]. Santoro et al., for instance, found that 5-, 8-, 9-, 11-HETE and 9-, 13-HODE significantly decrease insulin secretion in obese adolescents with T2DM [149]. Similarly, we have shown that metabolites generated by the conversion of LA to AA also impair GSIS by specifically downregulating ACSL4 expression, which subsequently results in the accumulation of intracellular CYP450 metabolites like EETs [100]. Our efforts to unveil the molecular mechanisms behind this process have also led to our recent discovery that LA and other PUFAs can reduce human ACSL4 transcription through PPAR response element (PPRE)-dependent transrepression of PPARα in vitro (unpublished data). Despite these new findings, additional studies are still needed to fully elucidate the exact role that LA plays in regulating insulin secretion and beta-cell function.
In summary, T2DM is a multifactorial disease defined by both insulin resistance in multiple tissues (skeletal muscle, adipocytes, and liver) and beta-cell dysfunction. There is a considerable body of evidence that increased dietary LA intake could help prevent the development of metabolic abnormalities, but the benefits may depend on an individual’s genetics and environment. To the contrary, it is possible that the increased consumption of LA could accelerate the pathogenesis of T2DM by decreasing insulin sensitivity and compromising insulin secretion from beta-cells. As hyperinsulinemia is the primary driver of insulin resistance, elevated plasma LA levels from the diet could potentially lead to systemic insulin resistance by disrupting the regulation and expression of key gluconeogenic genes in hepatic and peripheral tissues. Furthermore, the generation of OXLAMs and other oxylipins, either directly through the action of COXs, LOXs, and CYP450s or indirectly via the conversion of LA to AA, could also perpetuate intracellular oxidative stress and low-grade inflammation in the beta-cell that not only progressively disrupts GSIS, but induces beta-cell dysfunction. Given these possibilities, a reappraisal of optimal levels of dietary LA intake may be needed to determine what levels would more effectively reduce the incidence of T2DM and other chronic metabolic diseases.
[4]. Conclusions
Over the course of recent decades, our understanding of dietary fats has improved dramatically, but there is still much we do not yet understand about how PUFAs affect human health. This review summarizes the current knowledge concerning the dynamic physiological and pathophysiological roles of LA in the regulation of glucose homeostasis (Figure 2). By examining how LA affects insulin sensitivity and peripheral glucose uptake and insulin secretion in vitro and in vivo, we show through both animal models and human studies that the biological effects of dietary LA on glucose metabolism are both temporally- and dose-dependent and can be further modulated by numerous individual factors such as sex, genetics, environmental conditions, and disease status.
Figure 2:
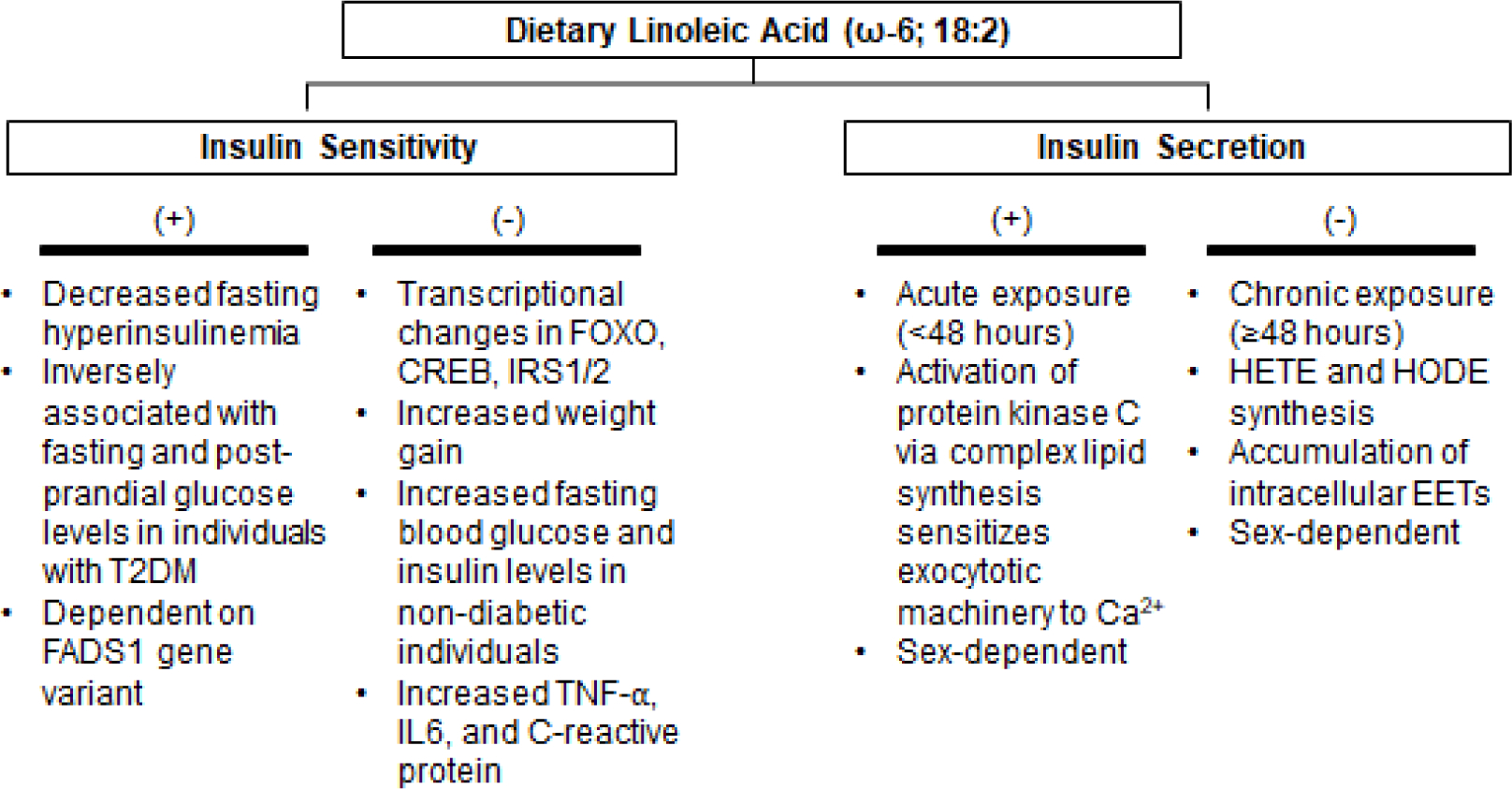
The Metabolic Effects of Linoleic Acid on Insulin Sensitivity and Secretion
It is known that small amounts of LA in the diet is essential, but it is becoming increasingly apparent that over consumption of LA can have deleterious effects. Clinical studies continue to show that different people consuming the same diets can exhibit divergent and sometimes unintended metabolic outcomes in terms of dietary responsiveness. As such, it is imperative that the field moves toward better defining the temporal and dose-dependent relationships associated with LA intake. A myriad of molecular and mechanistic research in cell and rodent models has advanced our understanding of the biological effects of various FAs, but some quantitative diet studies in animals have been a source of considerable confusion over the years. To address these confounders, quantitative and qualitative diet studies in humans would be the most appropriate to pursue. Such studies would not only clarify the critical aforementioned relationships concerning LA intake, but also provide insight on how human variability shapes the integration of dietary LA into health and disease.
Moving forward, it is now clear that the current trends in the consumption of LA are unparalleled and are largely a result of changes in dietary guidelines throughout the 20th century that were based on evidence generated by the SCS. While the substitution of SFAs with PUFAs like LA in the diet was recommended to reduce the incidence of CAD and CVD in Western countries, there are many tenets of the diet-heart hypothesis that remain contentious and are not easily translatable to the pathogenesis of other chronic metabolic diseases. A direct, yet unintended consequence of nutritional guidelines solely informed by the diet-heart hypothesis could be that the existing levels of dietary LA intake are compromising critical regulatory mechanisms in glucose metabolism by promoting insulin resistance in multiple tissues, and progressively reducing beta-cell insulin secretion. As a result, it is unmistakable that more effort should be taken to shift the paradigm on dietary fat consumption.
Regardless of what future findings may indicate on the relationship between heart disease, glucose regulation, and dietary LA intake, there is significant molecular and clinical evidence that the species-specific metabolic effects of LA and other dietary fats are dependent on the individual who consumes them. As such, the use of reductionist dietary recommendations that generalize the role of SFAs, MUFAs, and PUFAs in the body could be exacerbating, rather than alleviating, the current epidemic of chronic metabolic diseases.
Highlights.
Linoleic acid (LA, ω−6 18:2) is an essential fatty acid that accounted for 1–2% of total energy intake in the pre-industrial revolution human diet. Since the mid-20th century, the consumption of LA in Western civilizations has increased 10-fold in tandem with unprecedented incidence of chronic metabolic diseases like obesity and type 2 diabetes mellitus (T2DM).
Epidemiologic studies, meta-analyses, and clinical trials have outlined the potential benefits and unintended consequences of diets substituting SFAs with LA.
Well-controlled interventional randomized control trials are needed to better inform current nutritional guidelines and clarify how dietary LA and other individual ω−6 PUFAs affect insulin resistance and peripheral glucose uptake in individuals with and without T2DM.
Acknowledgements.
The authors would like to thank Drs. Rosalind A. Coleman and Shufen Chen for critical feedback and discussions. Further, we would like to thank Debbie and Brian Domeck for their financial support of our studies.
Funding:
This work was funded by National Institutes of Diabetes and Digestive and Kidney Diseases (grant DK107481 to E.L.K.).
The abbreviations used are:
- HPODE
13-hydroperoxyoctadecadienoic acid
- 13-HODE
13-hydroxyoctadecadienoic acid
- 9-HODE
9-hydroxyoctadecadienoic acid
- ACSL
acyl-CoA synthetase long-chain
- ADA
American Diabetes Association
- AHA
American Heart Association
- AA
arachidonic acid
- BAT
brown adipose tissue
- CREB
cAMP response element-binding protein
- CVD
cardiovascular disease
- CHARGE
Cohorts for Hearts and Aging Research in Genomic Epidemiology
- CAD
coronary artery disease
- COX
cyclooxygenase
- CYP450
cytochrome P450
- DiHOMEs
dihydroxyoctadecenoic acids
- EpOMEs
epoxyoctadecamonoenoic acids
- EPIC
European Prospective Investigation into Cancer and Nutrition
- FADS
fatty acid desaturase
- FORCE
Fatty Acids and Outcomes Research Consortium
- FOXO
forkhead box O
- GPR40
G-protein–coupled receptor 40
- GLUT
glucose transporter
- G6PC
glucose-6-phosphatase catalytic subunit
- GSIS
glucose-stimulated insulin secretion
- LA
linoleic acid
- LA-CoA
linoleoyl-CoA
- LOX
lipoxygenase
- mEHs
microsomal epoxide hydrolase
- MWD
Modern Western Diet
- MUFA
monounsaturated fatty acid
- NIH
National Institutes of Health
- NEFA
non-esterified fatty acid
- OcA
octanoic acid
- OA
oleic acid
- OXLAM
oxidized linoleic acid metabolites
- PA
palmitic acid
- PPAR
peroxisome proliferator-activated receptor
- PGC-1α
peroxisome proliferator-activated receptor gamma coactivator 1-alpha
- PPRE
peroxisome proliferator-activated receptor response element
- PCK
phosphoenolpyruvate carboxykinase
- PI3K
phosphoinositide 3-kinases
- PLA2
phospholipase A2
- PUFA
polyunsaturated fatty acid
- Akt
protein kinase B
- SFA
saturated fatty acid
- SCS
Seven Countries Study
- sEHs
soluble epoxide hydrolase
- SO
soybean oil
- SA
steric acid
- T2DM
type 2 diabetes mellitus
- USDA
United States Department of Agriculture
- HHS
United States Department of Health and Human Services
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Jaacks LM, Vandevijvere S, Pan A, McGowan CJ, Wallace C, Imamura F, Mozaffarian D, Swinburn B, Ezzati M. The obesity transition: stages of the global epidemic. Lancet Diabetes Endocrinol. 7 (2019) 231–240. 10.1016/S2213-8587(19)30026-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Blüher M Obesity: global epidemiology and pathogenesis. Nat Rev Endocrinol. 15 (2019) 288–298. 10.1038/s41574-019-0176-8. [DOI] [PubMed] [Google Scholar]
- [3].Savage DB, Petersen KF, Shulman GI. Disordered lipid metabolism and the pathogenesis of insulin resistance. Physiol Rev. 87 (2007) 507–520. 10.1152/physrev.00024.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Lewis GF, Carpentier A, Adeli K, Giacca A. Disordered fat storage and mobilization in the pathogenesis of insulin resistance and type 2 diabetes. Endocr Rev. 23 (2002) 201–229. 10.1210/edrv.23.2.0461. [DOI] [PubMed] [Google Scholar]
- [5].Cordain L, Eaton SB, Sebastian A, Mann N, Lindeberg S, Watkins BA, O’Keefe JH, Brand-Miller J. Origins and evolution of the Western diet: health implications for the 21st century. Am J Clin Nutr. 81(2005) 341–354. 10.1093/ajcn.81.2.341. [DOI] [PubMed] [Google Scholar]
- [6].(USDA) USDoA: Profiling Food Consumption in America. 2002, Washington, DC: Book AF [Google Scholar]
- [7].Kopp W How Western Diet And Lifestyle Drive The Pandemic Of Obesity And Civilization Diseases. Diabetes Metab Syndr Obes. 12 (2019) 2221–2236. 10.2147/DMSO.S216791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Sacks FM, Lichtenstein AH, Wu JHY, Appel LJ, Creager MA, Kris-Etherton PM, Miller M, Rimm EB, Rudel LL, Robinson JG, Stone NJ, Van Horn LV; American Heart Association. Dietary Fats and Cardiovascular Disease: A Presidential Advisory From the American Heart Association. Circulation. 136 (2017) e1–e23. 10.1161/CIR.0000000000000510. [DOI] [PubMed] [Google Scholar]
- [9].Dietary fat and its relation to heart attacks and strokes. Report by the Central Committee for Medical and Community Program of the American Heart Association. JAMA. 175 (1961) 389–391. [PubMed] [Google Scholar]
- [10].Harris WS, Mozaffarian D, Rimm E, Kris-Etherton P, Rudel LL, Appel LJ, Engler MM, Engler MB, Sacks F. Omega-6 fatty acids and risk for cardiovascular disease: a science advisory from the American Heart Association Nutrition Subcommittee of the Council on Nutrition, Physical Activity, and Metabolism; Council on Cardiovascular Nursing; and Council on Epidemiology and Prevention. Circulation. 119 (2009) 902–907. 10.1161/CIRCULATIONAHA. [DOI] [PubMed] [Google Scholar]
- [11].Oppenheimer GM, Benrubi ID. McGovern’s Senate Select Committee on Nutrition and Human Needs versus the meat industry on the diet-heart question (1976–1977). Am J Public Health. 104 (2014) 59–69. 10.2105/AJPH.2013.301464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].U.S. Department of Agriculture and U.S. Department of Health and Human Services. Dietary Guidelines for Americans, 2020–2025. 9th Edition. December 2020. Available at DietaryGuidelines.gov. [Google Scholar]
- [13].American Diabetes Association. 5. Lifestyle Management: Standards of Medical Care in Diabetes-2019. Diabetes Care. 42 (2019) S46–S60. 10.2337/dc19-S005. [DOI] [PubMed] [Google Scholar]
- [14].Lipid Research Clinics Coronary Primary Prevention Trial. (1984). The lipid research clinics coronary primary prevention trial results. (1984) American Medical Association; [DOI] [PubMed] [Google Scholar]
- [15].Keys A, Aravanis C, Blackburn HW, van Buchem SP, Buzina R, Djordjevic BS, Dontas AS, Fidanza F, Karvonen MJ, Kimura N, Lekos D, Monti M, Puddu V Taylor HL. Epidemiological studies related to coronary heart disease: Characteristics of men aged 40–59 in seven countries. Acta Med Scand. 460 (1966) 1–392. [PubMed] [Google Scholar]
- [16].Keys A Coronary heart disease in seven countries. Circulation. 41 (1970) 118–139. 10.1016/S0899-9007(96)00410-8. [DOI] [PubMed] [Google Scholar]
- [17].Keys A, Aravanis C, Blackburn H, Buzina R, Djordjevic BS, Dontas AS, Fidanza F, Karvonen MJ, Kimura N, Menotti A, Mohacek I, Nedeljkovic S, Pussu V, Punsar S, Taylor HL, Van Buchem FSP. Seven Countries: A Multivariate Analysis of Death and Coronary Heart Disease. Cambridge, MA: Harvard University Press; 1980. [Google Scholar]
- [18].Keys A, Menotti A, Karvonen MJ, et al. The diet and 15-year death rate in the seven countries study. Am J Epidemiol. 124 (1986) 903–915. 10.1093/oxfordjournals.aje.a114480. [DOI] [PubMed] [Google Scholar]
- [19].Keys A, Anderson JT, Grande F. Serum cholesterol response to changes in the diet: IV. Particular saturated fatty acids in the diet. Metabolism. 14 (1965) 776–787. 10.1016/0026-0495(65)90004-1. [DOI] [PubMed] [Google Scholar]
- [20].Keyes A, Anderson JT, Grande F. Serum cholesterol response to changes in the diet: I. Iodine value of dietary fat versus 2S-P. Metabolism. 14 (1965) 747–758. 10.1016/0026-0495(65)90001-6. [DOI] [PubMed] [Google Scholar]
- [21].Kinsell LW, Michaels GD, Walker G, Wheeler P, Splitter S, Flynn P. Dietary linoleic acid and linoleate: effects in diabetic and nondiabetic subjects with and without vascular disease. Diabetes. 8 (1959) 179–188. 10.2337/diab.8.3.179. [DOI] [PubMed] [Google Scholar]
- [22].Connor WE. Chemistry of the Lipides as Related to Atherosclerosis. AMA Arch Intern Med. 102 (1958) 336–337. 10.1001/archinte.1958.00260200164022. [DOI] [Google Scholar]
- [23].Pilkington TR, Stafford JL, Hankin VS, Simmonds FM, Koerselman HB. Practical Diets for Lowering Serum Lipids. Br Med J. 1 (1960) 23–25. 10.1136/bmj.1.5165.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Rose GA, Thomson WB, Williams RT. Corn Oil In Treatment Of Ischaemic Heart Disease. Br Med J. 1 (1965) 1531–1533. 10.1136/bmj.1.5449.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Leren P The effect of plasma cholesterol lowering diet in male survivors of myocardial infarction. A controlled clinical trial. Acta Med Scand Suppl. 466 (1966) 1–92. [PubMed] [Google Scholar]
- [26].Controlled trial of soya-bean oil in myocardial infarction. Lancet. 2 (1968) 693–699. 10.1016/S0140-6736(68)90746-0. [DOI] [PubMed] [Google Scholar]
- [27].Dayton S, Pearce ML. Diet high in unsaturated fat. A controlled clinical trial. Minn Med. 52 (1969) 1237–1242. 10.1161/01.cir.40.1s2.ii-1. [DOI] [PubMed] [Google Scholar]
- [28].Woodhill JM, Palmer AJ, Leelarthaepin B, McGilchrist C, Blacket RB. Low fat, low cholesterol diet in secondary prevention of coronary heart disease. Adv Exp Med Biol. 109 (1978) 317–330. 10.1007/978-1-4684-0967-3_18. [DOI] [PubMed] [Google Scholar]
- [29].Frantz ID Jr, Dawson EA, Ashman PL, Gatewood LC, Bartsch GE, Kuba K, Brewer ER. Test of effect of lipid lowering by diet on cardiovascular risk. The Minnesota Coronary Survey. Arteriosclerosis. 9 (1989) 129–135. 10.1161/01.atv.9.1.129. [DOI] [PubMed] [Google Scholar]
- [30].Leren P The Oslo diet-heart study. Eleven-year report. Circulation. 42 (1970) 935–942. 10.1161/01.cir.42.5.935. [DOI] [PubMed] [Google Scholar]
- [31].Turpeinen O, Karvonen MJ, Pekkarinen M, Miettinen M, Elosuo R, Paavilainen E. Dietary prevention of coronary heart disease: the Finnish Mental Hospital Study. Int J Epidemiol. 8 (1979) 99–118. 10.1093/ije/8.2.99. [DOI] [PubMed] [Google Scholar]
- [32].Ramsden CE, Zamora D, Majchrzak-Hong S, Faurot KR, Broste SK, Frantz RP, Davis JM, Ringel A, Suchindran CM, Hibbeln JR. Re-evaluation of the traditional diet-heart hypothesis: analysis of recovered data from Minnesota Coronary Experiment (1968–73). BMJ. 353 (2016) 10.1136/bmj.i1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Ramsden CE, Zamora D, Leelarthaepin B, Majchrzak-Hong SF, Faurot KR, Suchindran CM, Ringel A, Davis JM, Hibbeln JR. Use of dietary linoleic acid for secondary prevention of coronary heart disease and death: evaluation of recovered data from the Sydney Diet Heart Study and updated meta-analysis. BMJ. 346 (2013) e8707. 10.1136/bmj.e8707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Centers for Disease Control and Prevention (CDC). Decline in deaths from heart disease and stroke--United States, 1900–1999. MMWR Morb Mortal Wkly Rep. 48 (1999) 649–656. [PubMed] [Google Scholar]
- [35].Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW et al. American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics-2019 Update: A Report From the American Heart Association. Circulation. 139 (2019) e56–e528. 10.1161/CIR.0000000000000659. [DOI] [PubMed] [Google Scholar]
- [36].Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2020. Atlanta, GA: Centers for Disease Control and Prevention, U.S. Dept of Health and Human Services; 2020. [Google Scholar]
- [37].Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of obesity and severe obesity among adults: United States, 2017–2018. NCHS Data Brief, no 360. Hyattsville, MD: National Center for Health Statistics. 2020 [PubMed] [Google Scholar]
- [38].Zong G, Liu G, Willett WC, Wanders AJ, Alssema M, Zock PL, Hu FB, Sun Q. Associations Between Linoleic Acid Intake and Incident Type 2 Diabetes Among U.S. Men and Women. Diabetes Care. 42 (2019) 1406–1413. 10.2337/dc19-0412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Pertiwi K, Wanders AJ, Harbers MC, Küpers LK, Soedamah-Muthu SS, de Goede J, Zock PL, Geleijnse JM. Plasma and Dietary Linoleic Acid and 3-Year Risk of Type 2 Diabetes After Myocardial Infarction: A Prospective Analysis in the Alpha Omega Cohort. Diabetes Care. 43 (2020) 358–365. 10.2337/dc19-1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Alvheim AR, Malde MK, Osei-Hyiaman D, Lin YH, Pawlosky RJ, Madsen L, Kristiansen K, Frøyland L, Hibbeln JR. Dietary linoleic acid elevates endogenous 2-AG and anandamide and induces obesity. Obesity (Silver Spring). 20 (2012) 1984–1994. 10.1038/oby.2012.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Marangoni F, Agostoni C, Borghi C, Catapano AL, Cena H, Ghiselli A, La Vecchia C, Lercker G, Manzato E, Pirillo A, Riccardi G, Risé P, Visioli F, Poli A. Dietary linoleic acid and human health: Focus on cardiovascular and cardiometabolic effects. Atherosclerosis. 292 (2020) 90–98. 10.1016/j.atherosclerosis.2019.11.018. [DOI] [PubMed] [Google Scholar]
- [42].Blasbalg TL, Hibbeln JR, Ramsden CE, Majchrzak SF, Rawlings RR. Changes in consumption of omega-3 and omega-6 fatty acids in the United States during the 20th century. Am J Clin Nutr. 93 (2011) 950–962. 10.3945/ajcn.110.006643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Calder PC. Functional Roles of Fatty Acids and Their Effects on Human Health. JPEN J Parenter Enteral Nutr. 39 (2015) 18S–32S. 10.1177/0148607115595980. [DOI] [PubMed] [Google Scholar]
- [44].Patterson E, Wall R, Fitzgerald GF, Ross RP, Stanton C. Health implications of high dietary omega-6 polyunsaturated Fatty acids. J Nutr Metab. (2012) 539426. 10.1155/2012/539426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Lands B Historical perspectives on the impact of n-3 and n-6 nutrients on health. Prog Lipid Res. 55 (2014) 17–29. 10.1016/j.plipres.2014.04.002 [DOI] [PubMed] [Google Scholar]
- [46].Lands WE. Stories about acyl chains. Biochim Biophys Acta. 1483 (2000) 1–14. 10.1016/s1388-1981(99)00177-8. [DOI] [PubMed] [Google Scholar]
- [47].Lands B A critique of paradoxes in current advice on dietary lipids. Prog Lipid Res. 47 (2008) 77–106. 10.1016/j.plipres.2007.12.001. [DOI] [PubMed] [Google Scholar]
- [48].Wang X, Chan CB. n-3 polyunsaturated fatty acids and insulin secretion. J Endocrinol. 224 (2015) R97–106. 10.1530/JOE-14-0581. [DOI] [PubMed] [Google Scholar]
- [49].Simopoulos AP. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed Pharmacother. 56 (2002) 365–79. 10.1016/s0753-3322(02)00253-6. [DOI] [PubMed] [Google Scholar]
- [50].Raheja Bihari S., et al. “Significance of the n-6/n-3 ratio for insulin action in diabetes.” Annals of the New York Academy of Sciences 683 (1993) 258–271. [DOI] [PubMed] [Google Scholar]
- [51].de Lorgeril Michel, and Salen Patricia. “Modified Cretan Mediterranean diet in the prevention of coronary heart disease and cancer.” Mediterranean diets 87 (2000) 1–23 10.1159/000059721 [DOI] [PubMed] [Google Scholar]
- [52].Friday KE, Childs MT, Tsunehara CH, Fujimoto WY, Bierman EL, Ensinck JW. Elevated plasma glucose and lowered triglyceride levels from omega-3 fatty acid supplementation in type II diabetes. Diabetes Care. 12 (1989) 276–81. 10.2337/diacare.12.4.276. [DOI] [PubMed] [Google Scholar]
- [53].McEwen BJ, Morel-Kopp MC, Chen W, Tofler GH, Ward CM. Effects of omega-3 polyunsaturated fatty acids on platelet function in healthy subjects and subjects with cardiovascular disease. Semin Thromb Hemost. 39 (2013) 25–32. 10.1055/s-0032-1333309. [DOI] [PubMed] [Google Scholar]
- [54].Morris MC, Sacks F, Rosner B. Does fish oil lower blood pressure? A meta-analysis of controlled trials. Circulation. 88 (1993) 523–33. 10.1161/01.cir.88.2.523. [DOI] [PubMed] [Google Scholar]
- [55].U.S. Department of Agriculture, Agricultural Research Service. 2020. Snacks: Percentages of Selected Nutrients Contributed by Food and Beverages Consumed at Snack Occasions, by Race/Ethnicity and Age, What We Eat in America, NHANES 2017–2018.
- [56].Canada Health. “Do Canadian adults meet their nutrient requirements through food intake alone?.” Health Canada (2012): 978–981 [Google Scholar]
- [57].Ramírez-Silva I, Villalpando S, Moreno-Saracho JE, Bernal-Medina D. Fatty acids intake in the Mexican population. Results of the National Nutrition Survey 2006. Nutr Metab (Lond). 8 (2011) 33 10.1186/1743-7075-8-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Eilander A, Harika RK, Zock PL. Intake and sources of dietary fatty acids in Europe: Are current population intakes of fats aligned with dietary recommendations? Eur J Lipid Sci Technol. 117 (2015) 1370–1377. 10.1002/ejlt.201400513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Holman RT 1970. Biological activities of and requirements for polyunsaturated acids. p. 607–682 in Progress in the Chemistry of Fats and Other Lipids, Vol. 9. Pergamon Press, New York. [Google Scholar]
- [60].Smit EN, Muskiet FA, Boersma ER. The possible role of essential fatty acids in the pathophysiology of malnutrition: a review. Prostaglandins Leukot Essent Fatty Acids. 71 (2004) 241–250. 10.1016/j.plefa.2004.03.019. [DOI] [PubMed] [Google Scholar]
- [61].Simopoulos AP. Evolutionary aspects of diet, the omega-6/omega-3 ratio and genetic variation: nutritional implications for chronic diseases. Biomed Pharmacother. 60 (2006) 502–7. 10.1016/j.biopha.2006.07.080. Epub 2006 Aug 28. [DOI] [PubMed] [Google Scholar]
- [62].DeFronzo RA. Pathogenesis of type 2 diabetes mellitus. Med Clin North Am. 88 (2004) 787–835 10.1016/j.mcna.2004.04.013. [DOI] [PubMed] [Google Scholar]
- [63].Cersosimo E, Triplitt C, Solis-Herrera C, et al. Pathogenesis of Type 2 Diabetes Mellitus. [Updated 2018 Feb 27]. In: Feingold KR, Anawalt B, Boyce A, et al., editors. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK279115/. [Google Scholar]
- [64].Aronoff SL, Berkowitz K, Shreiner B, & Want L Glucose Metabolism and Regulation: Beyond Insulin and Glucagon. Diabetes Spectrum, 17 (2004) 183–190. 10.2337/diaspect.17.3.183. [DOI] [Google Scholar]
- [65].DeFronzo RA, Tripathy D. Skeletal muscle insulin resistance is the primary defect in type 2 diabetes. Diabetes Care. 32 (2009)S157–163. 10.2337/dc09-S302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Bansal P, Wang Q. Insulin as a physiological modulator of glucagon secretion. Am J Physiol Endocrinol Metab. 295 (2008) E751–761. 10.1152/ajpendo.90295.2008. [DOI] [PubMed] [Google Scholar]
- [67].MacDonald MJ, Fahien LA, Brown LJ, Hasan NM, Buss JD, Kendrick MA. Perspective: emerging evidence for signaling roles of mitochondrial anaplerotic products in insulin secretion. Am J Physiol Endocrinol Metab. 288 (2005) E1–15. 10.1152/ajpendo.00218.2004. [DOI] [PubMed] [Google Scholar]
- [68].Matschinsky FM, Wilson DF. The Central Role of Glucokinase in Glucose Homeostasis: A Perspective 50 Years After Demonstrating the Presence of the Enzyme in Islets of Langerhans. Front Physiol. 10 (2019) 10.3389/fphys.2019.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Popa Simona and Mota Maria (June 26th 2013). Beta-Cell Function and Failure in Type 2 Diabetes, Type 2 Diabetes, Kazuko Masuo, IntechOpen, DOI: 10.5772/56467. Available from: https://www.intechopen.com/books/type-2-diabetes/beta-cell-function-and-failure-in-type-2-diabetes#B1. [DOI] [Google Scholar]
- [70].McGarry JD, Dobbins RL. Fatty acids, lipotoxicity and insulin secretion. Diabetologia. 42 (1999) 128–138. 10.1007/s001250051130. [DOI] [PubMed] [Google Scholar]
- [71].Paolisso G, Gambardella A, Amato L, Tortoriello R, D’Amore A, Varricchio M, D’Onofrio F. Opposite effects of short- and long-term fatty acid infusion on insulin secretion in healthy subjects. Diabetologia. 38 (1995) 1295–1299. 10.1007/BF00401761. [DOI] [PubMed] [Google Scholar]
- [72].Carpentier A, Mittelman SD, Lamarche B, Bergman RN, Giacca A, Lewis GF. Acute enhancement of insulin secretion by FFA in humans is lost with prolonged FFA elevation. Am J Physiol. 276 (1999) E1055–66. 10.1152/ajpendo.1999.276.6.E1055. [DOI] [PubMed] [Google Scholar]
- [73].Amery CM, Round RA, Smith JM, Nattrass M. Elevation of plasma fatty acids by ten-hour intralipid infusion has no effect on basal or glucose-stimulated insulin secretion in normal man. Metabolism. 49 (2000) 450–454. 10.1016/s0026-0495(00)80007-4. [DOI] [PubMed] [Google Scholar]
- [74].Balent B, Goswami G, Goodloe G, Rogatsky E, Rauta O, Nezami R, Mints L, Angeletti RH, Stein DT. Acute elevation of NEFA causes hyperinsulinemia without effect on insulin secretion rate in healthy human subjects. Ann N Y Acad Sci. 967 (2002) 535–543. 10.1111/j.1749-6632.2002.tb04313.x. [DOI] [PubMed] [Google Scholar]
- [75].Santomauro A, Boden G, Silva M, Rocha DM, Santos RF, Ursich MJM, Strassman PG, Wajchenberg BL. Overnight lowering of free fatty acids with acipimox improves insulin resistance and glucose tolerance in obese diabetic and non-diabetic subjects. Diabetes. 48 (1999) 1836–1841. [DOI] [PubMed] [Google Scholar]
- [76].Kashyap S, Belfort R, Gastaldelli A, Pratipanawatr T, Berria R, Pratipanawatr W, Bajaj M, Mandarino L, DeFronzo R, Cusi K. A sustained increase in plasma free fatty acids impairs insulin secretion in nondiabetic subjects genetically predisposed to develop type 2 diabetes. Diabetes. 52 (2003) 2461–2474. 10.2337/diabetes.52.10.2461. [DOI] [PubMed] [Google Scholar]
- [77].Dobbins RL, Szczepaniak LS, Myhill J, Tamura Y, Uchino H, Giacca A, McGarry JD. The composition of dietary fat directly influences glucose-stimulated insulin secretion in rats. Diabetes. 51 (2002) 1825–33. 10.2337/diabetes.51.6.1825. [DOI] [PubMed] [Google Scholar]
- [78].Nunes E, Peixoto F, Louro T, Sena CM, Santos MS, Matafome P, Moreira PI, Seiça R. Soybean oil treatment impairs glucose-stimulated insulin secretion and changes fatty acid composition of normal and diabetic islets. Acta Diabetol. 44 (2007) 121–130. 10.1007/s00592-007-0252-8. [DOI] [PubMed] [Google Scholar]
- [79].Xiao C, Giacca A, Carpentier A, Lewis GF. Differential effects of monounsaturated, polyunsaturated and saturated fat ingestion on glucose-stimulated insulin secretion, sensitivity and clearance in overweight and obese, non-diabetic humans. Diabetologia. 49 (2006) 1371–1379. 10.1007/s00125-006-0211-x. [DOI] [PubMed] [Google Scholar]
- [80].Klett EL, Chen S, Yechoor A, Lih FB, Coleman RA. Long-chain acyl-CoA synthetase isoforms differ in preferences for eicosanoid species and long-chain fatty acids. J Lipid Res. 58 (2017) 884–894. 10.1194/jlr.M072512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Soupene E, Kuypers FA. Mammalian long-chain acyl-CoA synthetases. Exp Biol Med (Maywood). 233 (2008) 507–521. 10.3181/0710-MR-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Grevengoed TJ, Klett EL, Coleman RA. Acyl-CoA metabolism and partitioning. Annu Rev Nutr. 34 (2014) 1–30. 10.1146/annurev-nutr-071813-105541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Ramsden CE, Ringel A, Feldstein AE, Taha AY, MacIntosh BA, Hibbeln JR, Majchrzak-Hong SF, Faurot KR, Rapoport SI, Cheon Y, Chung YM, Berk M, Mann JD. Lowering dietary linoleic acid reduces bioactive oxidized linoleic acid metabolites in humans. Prostaglandins Leukot Essent Fatty Acids. 87 (2012) 135–141. 10.1016/j.plefa.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Kwon SY, Massey K, Watson MA, Hussain T, Volpe G, Buckley CD, Nicolaou A, Badenhorst P. Oxidised metabolites of the omega-6 fatty acid linoleic acid activate dFOXO. Life Sci Alliance. 3 (2020) e201900356. 10.26508/lsa.201900356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Ozawa T, Nishikimi M, Sugiyama S, Taki F, Hayakawa M, & Shionoya H Cytotoxic activity of leukotoxin, a neutrophil-derived fatty acid epoxide, on cultured human cells. Biochemistry international, 16 (1988) 369–373. [PubMed] [Google Scholar]
- [86].Gabbs M, Leng S, Devassy JG, Monirujjaman M, Aukema HM. Advances in Our Understanding of Oxylipins Derived from Dietary PUFAs. Adv Nutr. 6 (2015) 513–40. 10.3945/an.114.007732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Sonnweber T, Pizzini A, Nairz M, Weiss G, Tancevski I. Arachidonic Acid Metabolites in Cardiovascular and Metabolic Diseases. Int J Mol Sci. 19 (2018) 3285. 10.3390/ijms19113285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Kuwata H, Hara S. Role of acyl-CoA synthetase ACSL4 in arachidonic acid metabolism. Prostaglandins Other Lipid Mediat. 144 (2019. 106363. 10.1016/j.prostaglandins.2019.106363. [DOI] [PubMed] [Google Scholar]
- [89].Wang Weicang, Zhang Jianan, Zhang Guodong, Cytochrome P450 monooxygenase-mediated eicosanoid pathway: A potential mechanistic linkage between dietary fatty acid consumption and colon cancer risk, Food Science and Human Wellness 8 (2019) 337–343 10.1016/j.fshw.2019.11.002. [DOI] [Google Scholar]
- [90].Moghaddam MF, Grant DF, Cheek JM, Greene JF, Williamson KC, & Hammock BD Bioactivation of leukotoxins to their toxic diols by epoxide hydrolase. Nature medicine, 3 (1997), 562–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Hildreth K, Kodani SD, Hammock BD, Zhao L. Cytochrome P450-derived linoleic acid metabolites EpOMEs and DiHOMEs: a review of recent studies. J Nutr Biochem. 86 (2020) 108484. 10.1016/j.jnutbio.2020.108484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Sarparast M, Dattmore D, Alan J, Lee KSS. Cytochrome P450 Metabolism of Polyunsaturated Fatty Acids and Neurodegeneration. Nutrients. 12 (2020) 3523. 10.3390/nu12113523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Gautheron J, Jéru I. The Multifaceted Role of Epoxide Hydrolases in Human Health and Disease. Int J Mol Sci. 22 (2020) 13. 10.3390/ijms22010013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Lynes MD, Leiria LO, Lundh M, Bartelt A, Shamsi F, Huang TL, et al. The cold-induced lipokine 12,13-diHOME promotes fatty acid transport into brown adipose tissue. Nat Med. 23 (2017) 631–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Hussein N, Ah-Sing E, Wilkinson P, Leach C, Griffin BA, Millward DJ. Long-chain conversion of [13C]linoleic acid and alpha-linolenic acid in response to marked changes in their dietary intake in men. J Lipid Res. 46 (2005) 269–280. 10.1194/jlr.M400225-JLR200. [DOI] [PubMed] [Google Scholar]
- [96].Leng SM and Aukema HM (2016), Dietary Linoleic Acid (LA) Increases Linoleic and Arachidonic Acid (ARA) Derived Bioactive Lipids, Despite Not Altering Tissue Fatty Acid Levels. The FASEB Journal, 30: 130.7–130.7. [Google Scholar]
- [97].Angela Liou Y, Sheila M Innis, Dietary linoleic acid has no effect on arachidonic acid, but increases n-6 eicosadienoic acid, and lowers dihomo-γ-linolenic and eicosapentaenoic acid in plasma of adult men, Prostaglandins Leukot Essent Fatty Acids. 80 (2009) 201–206 10.1016/j.plefa.2009.02.003. [DOI] [PubMed] [Google Scholar]
- [98].Rett BS, Whelan J. Increasing dietary linoleic acid does not increase tissue arachidonic acid content in adults consuming Western-type diets: a systematic review. Nutr Metab (Lond). 10 (2011) 8–36. 10.1186/1743-7075-8-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Innes JK, Calder PC. Omega-6 fatty acids and inflammation. Prostaglandins Leukot Essent Fatty Acids. 132 (2018) 41–48. 10.1016/j.plefa.2018.03.004. [DOI] [PubMed] [Google Scholar]
- [100].Klett EL, Chen S, Edin ML, Li LO, Ilkayeva O, Zeldin DC, Newgard CB, Coleman RA. Diminished acyl-CoA synthetase isoform 4 activity in INS 832/13 cells reduces cellular epoxyeicosatrienoic acid levels and results in impaired glucose-stimulated insulin secretion. J Biol Chem. 288 (2013) 21618–29. 10.1074/jbc.M113.481077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Inazumi T, Yamada K, Shirata N, Sato H, Taketomi Y, Morita K, et al. Prostaglandin E2-EP4 Axis Promotes Lipolysis and Fibrosis in Adipose Tissue Leading to Ectopic Fat Deposition and Insulin Resistance. Cell Rep. 33 (2020) 108265. 10.1016/j.celrep.2020.108265. [DOI] [PubMed] [Google Scholar]
- [102].Lee ML., Matsunaga H, Sugiura Y et al. Prostaglandin in the ventromedial hypothalamus regulates peripheral glucose metabolism. Nat Commun. 12 (2021) 2330 10.1038/s41467-021-22431-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Thorp AA, Schlaich MP. Relevance of Sympathetic Nervous System Activation in Obesity and Metabolic Syndrome. J Diabetes Res. (2015) 341583. 10.1155/2015/341583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2021. Diabetes Care. 44 (2021) S15–S33. 10.2337/dc21-S002. [DOI] [PubMed] [Google Scholar]
- [105].Cabout M, Alssema M, Nijpels G, Stehouwer CDA, Zock PL, Brouwer IA, Elshorbagy AK, Refsum H, Dekker JM. Circulating linoleic acid and alpha-linolenic acid and glucose metabolism: the Hoorn Study. Eur J Nutr. 56 (2017) 2171–2180. 10.1007/s00394-016-1261-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Lankinen MA, Fauland A, Shimizu BI, Ågren J, Wheelock CE, Laakso M, Schwab U, Pihlajamäki J. Inflammatory response to dietary linoleic acid depends on FADS1 genotype. Am J Clin Nutr. 109 (2019) 165–175. 10.1093/ajcn/nqy287. [DOI] [PubMed] [Google Scholar]
- [107].Mathias RA, Pani V, Chilton FH. Genetic Variants in the FADS Gene: Implications for Dietary Recommendations for Fatty Acid Intake. Curr Nutr Rep. 3 (2014) 139–148. 10.1007/s13668-014-0079-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].DeFronzo RA, Gunnarsson R, Björkman O, Olsson M, Wahren J. Effects of insulin on peripheral and splanchnic glucose metabolism in noninsulin-dependent (type II) diabetes mellitus. J Clin Invest. 76 (1985) 149–55. 10.1172/JCI111938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Hatting M, Tavares CDJ, Sharabi K, Rines AK, Puigserver P. Insulin regulation of gluconeogenesis. Ann N Y Acad Sci. 1411 (2018) 21–35. 10.1111/nyas.13435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Barthel A, Schmoll D. Novel concepts in insulin regulation of hepatic gluconeogenesis. Am J Physiol Endocrinol Metab. 285 (2003) E685–92. 10.1152/ajpendo.00253.2003. [DOI] [PubMed] [Google Scholar]
- [111].Kobayashi MASASHI, and Olefsky JERROLDM. “Effect of experimental hyperinsulinemia on insulin binding and glucose transport in isolated rat adipocytes.” American Journal of Physiology-Endocrinology and Metabolism 235.1 (1978): E53. [DOI] [PubMed] [Google Scholar]
- [112].Martin C, Desai KS, Steiner G. Receptor and postreceptor insulin resistance induced by in vivo hyperinsulinemia. Can J Physiol Pharmacol. 61 (1983) 802–807. 10.1139/y83-123. [DOI] [PubMed] [Google Scholar]
- [113].Rizza RA, Mandarino LJ, Genest J, Baker BA, Gerich JE. Production of insulin resistance by hyperinsulinaemia in man. Diabetologia. 28 (1985) 70–75. 10.1007/BF00279918. [DOI] [PubMed] [Google Scholar]
- [114].Shanik MH, Xu Y, Skrha J, Dankner R, Zick Y, Roth J. Insulin resistance and hyperinsulinemia: is hyperinsulinemia the cart or the horse? Diabetes Care. 31 (2008) S262–8. 10.2337/dc08-s264. [DOI] [PubMed] [Google Scholar]
- [115].Wu JHY, Marklund M, Imamura F, Tintle N, Ardisson Korat AV, de Goede J, et al. Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) Fatty Acids and Outcomes Research Consortium (FORCE). Omega-6 fatty acid biomarkers and incident type 2 diabetes: pooled analysis of individual-level data for 39 740 adults from 20 prospective cohort studies. Lancet Diabetes Endocrinol. 5 (2017) 965–974. 10.1016/S2213-8587(17)30307-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Forouhi NG, Imamura F, Sharp SJ, Koulman A, Schulze MB, Zheng J, et al. Association of Plasma Phospholipid n-3 and n-6 Polyunsaturated Fatty Acids with Type 2 Diabetes: The EPIC-InterAct Case-Cohort Study. PLoS Med. 13 (2016) e1002094. 10.1371/journal.pmed.1002094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Mousavi SM, Jalilpiran Y, Karimi E et al. Dietary Intake of Linoleic Acid, Its Concentrations, and the Risk of Type 2 Diabetes: A Systematic Review and Dose-Response Meta-analysis of Prospective Cohort Studies. Diabetes Care 44 (2021) 2173–2181. 10.2337/dc21-0438. [DOI] [PubMed] [Google Scholar]
- [118].Zong G, Liu G, Willett WC, et al. Associations between linoleic acid intake and incident type 2 diabetes among U.S. men and women. Diabetes Care 42 (2019) 1406–1413 10.2337/dc19-0412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Schulze MB. Dietary Linoleic Acid: Will Modifying Dietary Fat Quality Reduce the Risk of Type 2 Diabetes? Diabetes Care 44 (2021) 1913–1915. 10.2337/dci21-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Imamura F, Micha R, Wu JH, de Oliveira Otto MC, Otite FO, Abioye AI, Mozaffarian D. Effects of Saturated Fat, Polyunsaturated Fat, Monounsaturated Fat, and Carbohydrate on Glucose-Insulin Homeostasis: A Systematic Review and Meta-analysis of Randomised Controlled Feeding Trials. PLoS Med. 13 (2016) e1002087. 10.1371/journal.pmed.1002087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Belury MA, Cole RM, Bailey BE, Ke JY, Andridge RR, Kiecolt-Glaser JK. Erythrocyte linoleic acid, but not oleic acid, is associated with improvements in body composition in men and women. Mol Nutr Food Res. 60 (2016) 1206–1212. 10.1002/mnfr.201500744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Wilcox G. Insulin and insulin resistance. Clin Biochem Rev. 25 (2005) 19–39. [PMC free article] [PubMed] [Google Scholar]
- [123].Mack R, Skurnick B, Sterling-Jean Y, Pedra-Nobre M, Bigg D. Fasting insulin levels as a measure of insulin resistance in American blacks. J Med. 34 (2003) 31–38. [PubMed] [Google Scholar]
- [124].Weyer C, Hanson RL, Tataranni PA, Bogardus C, Pratley RE. A high fasting plasma insulin concentration predicts type 2 diabetes independent of insulin resistance: evidence for a pathogenic role of relative hyperinsulinemia. Diabetes. 49 (2000) 2094–2101. 10.2337/diabetes.49.12.2094. [DOI] [PubMed] [Google Scholar]
- [125].Deol P, Evans JR, Dhahbi J, Chellappa K, Han DS, Spindler S, Sladek FM. Soybean Oil Is More Obesogenic and Diabetogenic than Coconut Oil and Fructose in Mouse: Potential Role for the Liver. PLoS One. 10 (2015) e0132672. 10.1371/journal.pone.0132672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Clemente TE, Cahoon EB. Soybean oil: genetic approaches for modification of functionality and total content. Plant Physiol. 151 (2009) 1030–1040. 10.1104/pp.109.146282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Pei Xinli, Xie Zhongguo, Wang Jing, Shi Kaiwen, Han Fei, Li Aike & Liu Haiying (2018) The effect of various intake levels of soybean oil on blood glucose and inflammation in mice, Food and Agricultural Immunology 29 (2018) 511–523. 10.1080/09540105.2017.1409194. [DOI] [Google Scholar]
- [128].Gu Z, Mu H, Shen H, Deng K, Liu D, Yang M, Zhang Y, Zhang W, Mai K. High level of dietary soybean oil affects the glucose and lipid metabolism in large yellow croaker Larimichthys crocea through the insulin-mediated PI3K/AKT signaling pathway. Comp Biochem Physiol B Biochem Mol Biol. 231 (2019) 34–41. 10.1016/j.cbpb.2018.12.003. [DOI] [PubMed] [Google Scholar]
- [129].Mohammadpour F, Darmani-Kuhi H, Mohit A, Sohani MM. Obesity, insulin resistance, adiponectin, and PPAR-γ gene expression in broiler chicks fed diets supplemented with fat and green tea (Camellia sinensis) extract. Domest Anim Endocrinol. (2020) 106440. 10.1016/j.domaniend.2020.106440. [DOI] [PubMed] [Google Scholar]
- [130].Nowotny B, Zahiragic L, Krog D, Nowotny PJ, Herder C, Carstensen M, Yoshimura T, Szendroedi J, Phielix E, Schadewaldt P, Schloot NC, Shulman GI, Roden M. Mechanisms underlying the onset of oral lipid-induced skeletal muscle insulin resistance in humans. Diabetes. 62 (2013) 2240–2248. 10.2337/db12-1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Naughton SS, Hanson ED, Mathai ML, McAinch AJ. The Acute Effect of Oleic- or Linoleic Acid-Containing Meals on Appetite and Metabolic Markers; A Pilot Study in Overweight or Obese Individuals. Nutrients. 10 (2018) 1376. 10.3390/nu10101376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Kim M, Kim M, Lee A, Yoo HJ, Her JS, Jee SH, Lee JH. Impact of 8-week linoleic acid intake in soy oil on Lp-PLA2 activity in healthy adults. Nutr Metab (Lond). (2017) 14–32 10.1186/s12986-017-0186-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Sung KC, Hwang ST. Association between insulin resistance and apolipoprotein B in normoglycemic Koreans. Atherosclerosis. 180 (2005) 161–169. 10.1016/j.atherosclerosis.2004.11.009. [DOI] [PubMed] [Google Scholar]
- [134].Zulkiply SH, Balasubramaniam V, Abu Bakar NA, Abd Rashed A, Ismail SR. Effects of palm oil consumption on biomarkers of glucose metabolism: A systematic review. PLoS One. 14 (2019) e0220877. 10.1371/journal.pone.0220877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Frantz ID Jr, Dawson EA, Ashman PL, Gatewood LC, Bartsch GE, Kuba K, Brewer ER. Test of effect of lipid lowering by diet on cardiovascular risk. The Minnesota Coronary Survey. Arteriosclerosis. 9 (1989) 129–35. 10.1161/01.atv.9.1.129. [DOI] [PubMed] [Google Scholar]
- [136].Woodhill JM, Palmer AJ, Leelarthaepin B, McGilchrist C, Blacket RB. Low fat, low cholesterol diet in secondary prevention of coronary heart disease. Adv Exp Med Biol. 109 (1978) 317–330. 10.1007/978-1-4684-0967-3_18. [DOI] [PubMed] [Google Scholar]
- [137].Hudish LI, Reusch JE, Sussel L. β Cell dysfunction during progression of metabolic syndrome to type 2 diabetes. J Clin Invest. 129 (2019) 4001–4008. 10.1172/JCI129188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Pelikánová T, Kohout M, Válek J, Kazdová L, Karasová L, Base J, Stefka Z. Sekrece inzulínu a inzulínová rezistence u diabetu II. typu. Vztahy ve slození mastných kyselin ve fosfolipidech séra [Insulin secretion and insulin resistance in type II diabetes. Relation to fatty acid composition of serum phospholipids]. Cas Lek Cesk. 129 (1990) 1605–1610. [PubMed] [Google Scholar]
- [139].Gravena C, Mathias PC, Ashcroft SJ. Acute effects of fatty acids on insulin secretion from rat and human islets of Langerhans. J Endocrinol. 173 (2002) 73–80. 10.1677/joe.0.1730073. [DOI] [PubMed] [Google Scholar]
- [140].Itoh Y, Kawamata Y, Harada M et al. Free fatty acids regulate insulin secretion from pancreatic β cells through GPR40. Nature 422, (2003) 173–176. 10.1038/nature01478. [DOI] [PubMed] [Google Scholar]
- [141].Kashyap S, Belfort R, Gastaldelli A, Pratipanawatr T, Berria R, Pratipanawatr W, Bajaj M, Mandarino L, DeFronzo R, Cusi K. A sustained increase in plasma free fatty acids impairs insulin secretion in nondiabetic subjects genetically predisposed to develop type 2 diabetes. Diabetes. 52 (2003) 2461–2474. 10.2337/diabetes.52.10.2461. [DOI] [PubMed] [Google Scholar]
- [142].Nemati R, Lu J, Tura A, Smith G, Murphy R. Acute Changes in Non-esterified Fatty Acids in Patients with Type 2 Diabetes Receiving Bariatric Surgery. Obes Surg. 27 (2017) 649–656. 10.1007/s11695-016-2323-9. [DOI] [PubMed] [Google Scholar]
- [143].Basu A, Dube S, Basu R. Men Are from Mars, Women Are from Venus: Sex Differences in Insulin Action and Secretion. Adv Exp Med Biol. 1043 (2017) 53–64. 10.1007/978-3-319-70178-3_4. [DOI] [PubMed] [Google Scholar]
- [144].Hart LM, Fritsche A, Rietveld I, Dekker JM, Nijpels G, Machicao F, Stumvoll M, van Duijn CM, Häring HU, Heine RJ, Maassen JA, van Haeften TW. Genetic factors and insulin secretion: gene variants in the IGF genes. Diabetes. 53 (2004) S26–30. 10.2337/diabetes.53.2007.s26 [DOI] [PubMed] [Google Scholar]
- [145].Dover EN, Beck R, Huang MC, Douillet C, Wang Z, Klett EL, Stýblo M. Arsenite and methylarsonite inhibit mitochondrial metabolism and glucose-stimulated insulin secretion in INS-1 832/13 β cells. Arch Toxicol. 92 (2018) 693–704. 10.1007/s00204-017-2074-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Lai MC, Teng TH, Yang C. The natural PPAR agonist linoleic acid stimulated insulin release in the rat pancreas. J Vet Med Sci. 75 (2013) 1449–1454. 10.1292/jvms.13-0189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Zhou YJ, Song YL, Zhou H, Li Y. Linoleic acid activates GPR40/FFA1 and phospholipase C to increase [Ca2+]i release and insulin secretion in islet beta-cells. Chin Med Sci J. 27 (2012) 18–23. 10.1016/s1001-9294(12)60017-0. [DOI] [PubMed] [Google Scholar]
- [148].Itoh Y, Kawamata Y, Harada M, Kobayashi M, Fujii R, Fukusumi S, Ogi K, Hosoya M, Tanaka Y, Uejima H, Tanaka H, Maruyama M, Satoh R, Okubo S, Kizawa H, Komatsu H, Matsumura F, Noguchi Y, Shinohara T, Hinuma S, Fujisawa Y, Fujino M. Free fatty acids regulate insulin secretion from pancreatic beta cells through GPR40. Nature. 422 (2003) 173–176. 10.1038/nature01478. [DOI] [PubMed] [Google Scholar]
- [149].Santoro N, Caprio S, Giannini C, Kim G, Kursawe R, Pierpont B, Shaw MM, Feldstein AE. Oxidized fatty acids: A potential pathogenic link between fatty liver and type 2 diabetes in obese adolescents? Antioxid Redox Signal. 20 (2014) 383–389. 10.1089/ars.2013.5466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Yaney GC, Corkey BE. Fatty acid metabolism and insulin secretion in pancreatic beta cells. Diabetologia. 46 (2003) 1297–312. 10.1007/s00125-003-1207-4. [DOI] [PubMed] [Google Scholar]
- [151].Dos Santos LRB, Fleming I. Role of cytochrome P450-derived, polyunsaturated fatty acid mediators in diabetes and the metabolic syndrome. Prostaglandins Other Lipid Mediat. 148 (2020) 106407. 10.1016/j.prostaglandins.2019.106407. [DOI] [PubMed] [Google Scholar]


