Abstract
The Covid-19 pandemic has caused millions of deaths worldwide. Although vaccines have been developed, patients on immunosuppressive therapy are less likely to respond. This study was aimed at investigating the efficacy of a Covid-19 vaccine (Pfizer-BioNTech) in patients with non-Hodgkin lymphoma treated with anti-CD20 monoclonal antibodies. Only 1 of 28 lymphoma patients (3.6%) developed a seropositive response, compared with 100% (28/28) of the healthy volunteers. The low levels of CD19+ lymphocytes among the lymphoma patients suggest that anti-CD20 treatment prevents the seropositive response to the vaccine. An additional vaccination might be indicated in these patients once B cells are repopulated.
The novel coronavirus Covid-19 has afflicted millions of people worldwide. Several vaccines have been developed to contain the pandemic. The BNT162b2 vaccine (BioNTech, Pfizer vaccine), tested in 43,548 participants, was reported to provide 95% protection against Covid-19 [1]. Comparable results were achieved in a phase III trial testing the mRNA-1273 (Moderna, NIAID) vaccine [2]. On the basis of these results, BNT162b2 and mRNA-1273 received global approval, and individuals are being vaccinated worldwide.
Patients with an immunocompromising condition or on immunosuppressive therapy were excluded in the aforementioned trials, and there are no data regarding the efficacy of the vaccine in this population. A concerning subgroup comprises patients treated with anti-CD20 monoclonal antibodies (mAbs). These agents have been incorporated as standard of care in the treatment of CD20+ B-cell lymphomas. Anti-CD20 mAbs deplete normal B cells and thus impair humoral response.
In a study of lymphoma patients who received a rituximab-containing treatment regimen within the previous 6 months, none of the 67 patients developed seroprotective titers after administration of an adjuvanted, inactivated pandemic H1N1 influenza A vaccine. In contrast, 42 of 51 healthy controls (82%) developed seroprotective titers after vaccination [3]. Nonetheless, the guidelines of the Israel Health Ministry, based on expert opinion, recommend vaccination of patients who receive immunosuppressive therapy [4].
The aim of this study was to determine the efficacy of the Covid-19 vaccine in hematological patients treated with anti-CD20 mAbs during the 6 months before vaccination. Of additional interest was assessment of a correlation between B-cell depletion and the efficacy of the vaccine.
Methods
Patients eligible for the study were adults with an established diagnosis of non-Hodgkin lymphoma (NHL) receiving anti-CD20 mAbs as a single agent or in combination with chemotherapy. Patients not on active treatment were eligible if treatment with anti-CD20 mAbs had been completed within the last 6 months before the first vaccination. Controls were volunteers without hematological disease. Patients with chronic lymphocytic leukemia/small lymphocytic lymphoma and patients or volunteers with previous laboratory-confirmed Covid-19 infection were excluded.
All participants received two Covid-19 vaccines (Pfizer-BioNTech) 21 days apart. Between days 7 and 72 after the second vaccination, blood samples were drawn for Covid-19 serology. The antibody response to vaccine was measured using the Abbott Architect SARS-Cov-2 IgG II immunoassay (Abbott Laboratories, Abbott Park, IL) targeting Spike IgG. A level of 150 AU/mL was considered positive. Patients with a positive Spike IgG were subsequently tested with the Abbott Architect SARS-CoV-2 IgG assay targeting nucleocapsid to rule out past infection with Covid-19. Additional laboratory parameters evaluated included complete blood count, immunoglobulin levels, and peripheral blood lymphocyte subset analysis measured by flow cytometry. Markers for B cells were CD19 and CD40; for memory B cells, CD27; and for T cells, CD3, CD4, and CD8. The study was approved by the ethics committee of Shaare Zedek Medical Center (Jerusalem, Israel). All participants gave written informed consent.
Results and discussion
Twenty-eight NHL patients and 28 controls were recruited for this study. Patient characteristics and clinical data are summarized in Table 1 . The controls included 22 female and 6 male volunteers with a median age of 50 years (range: 27–75). Most patients received combined treatment with chemotherapy and anti-CD20 antibody, 3 patients received rituximab as a single agent, and 12 received rituximab as maintenance therapy.
Table 1.
Patient characteristics and clinical data (n = 28)
| Characteristics | Value |
|---|---|
| Median age (years) [4] | 69 (54–94)* |
| Female/male | 8/20 |
| Therapy | |
| Rituximab monotherapy | 3 |
| Rituximab maintenance† | 12 |
| Bendamustine–rituximab | 3 |
| Bendamustine–obinutuzumab | 2 |
| R-CHOP | 8 |
| Type of Non-Hodgkin lymphoma | |
| Diffuse large B-cell lymphoma | 8 |
| Follicular lymphoma | 14 |
| Marginal zone lymphoma | 6 |
| Treatment | |
| Time from last mAbs dose to first vaccination (days) | 25 (9–162) |
| No. of therapy cycles in latest treatment line before vaccination | 9 (3–19) |
| Treatment (primary/salvage) | 27/1 |
| Laboratory data | |
| White blood cell count (× 10³/µL) | 4.700 (2.200–8.600) |
| Absolute lymphocyte count (× 10³/µL) | 0.96 (0.22–2.24) |
| Immunoglobulin level (mg/dL) | |
| IgG | 866 (338–1340) |
| IgA | 118 (<27 to 271) |
| IgM | 26 (<17 to 796) |
| Immunophenotyping | |
| CD3 count (% of lymphocytes) | 81 (51–98) |
| CD4 count (% of CD3+ cells) | 41 (16–76) |
| CD8 count (% of CD3+ cells) | 49 (22–78) |
| CD19 count (% of lymphocytes) | 0 (0–12) |
CHOP=cyclophosphamide, doxorubicin, vincristine, and prednisone; mAbs=monoclonal antibodies; R=rituximab.
Values are expressed as the number of patients or median (range).
Four patients after R-CHOP, one patient after R-CVP, seven patients after bendamustine–rituximab.
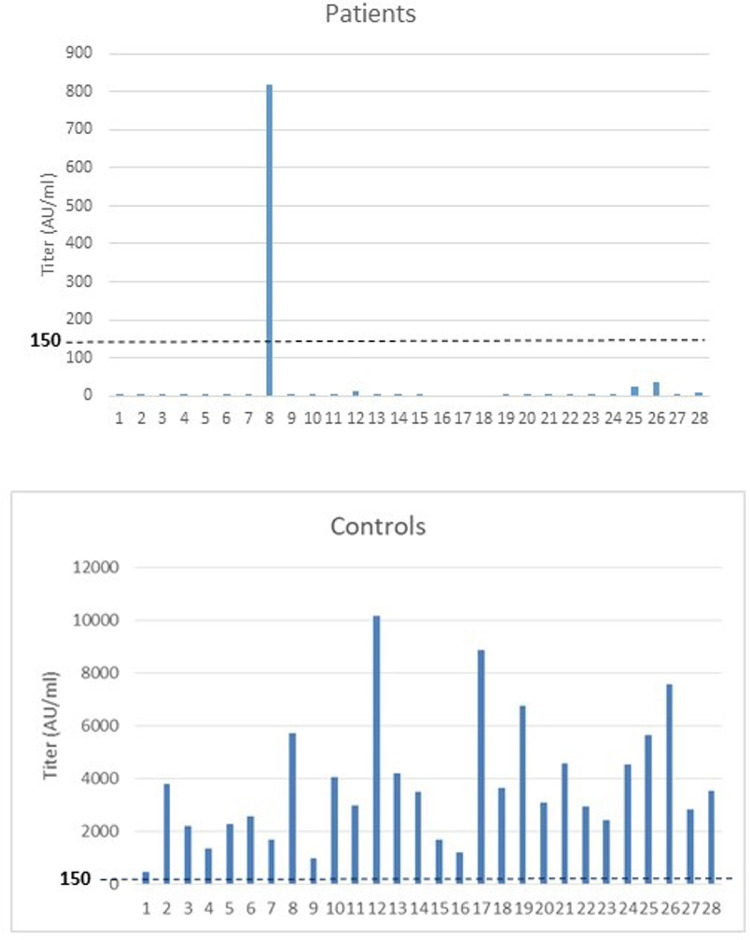
Among the 28 lymphoma patients, only one patient developed a seropositive response after Covid-19 vaccination. In contrast, all the controls had high SARS-Cov-2 IgG levels (Figure 1 ). This yields a seropositive rate of 3.6% in patients versus 100% in controls. Low levels of at least one class of immunoglobulins were observed in 16 patients (57%). CD3⁺, CD4⁺, and CD 8⁺ cell counts were within normal ranges in all patients. No CD19+ lymphocytes were detected in 27 of 28 patients.
Figure 1.
SARS-Cov-2 IgG levels after Covid-19 vaccination in patients after treatment with monoclonal anti-CD20 antibody and controls.
In the current study, almost all lymphoma patients treated with anti-CD20 mAbs alone or in combination with chemotherapy failed to exhibit a seropositive response after Covid-19 vaccination. CD19 count was low in 27 of 28 lymphoma patients. During a follow-up period of 3 months, only one of these patients developed a mild Covid-19 infection.
One NHL patient had a seropositive result after Covid-19 vaccination. This 80-year-old patient had been diagnosed with Helicobacter pylori-negative gastric marginal zone lymphoma, stage I. He received four courses of single-agent rituximab once weekly and four additional courses once monthly, the last 2 months before he was vaccinated for Covid-19. His immunoassay for spike protein was positive. No antibodies against SARS-Cov-2 nucleocapsid protein were detected, implying no prior Covid-19 infection. His CD19 lymphocyte count was zero. Lymphocyte count and immunoglobulins were within normal ranges. There was no apparent explanation why this patient, in contrast to all the other NHL patients, developed a seropositive response. Eight months after his last rituximab treatment, the patient received his third vaccination for Covid-19. A month later he had sustained a seropositive response. CD19+ lymphocytes were detected.
In a study assessing humoral response to influenza vaccination in patients with NHL, patients not needing treatment exhibited a good serological response to H1N1 vaccinations [5]. In the same study, patients treated with chemotherapy without rituximab also had a sufficient immune response, although not as good as that in healthy controls. In contrast, Yri et al. [3] describes 67 lymphoma patients treated with rituximab, none of whom had a seropositive result after H1N1 vaccination, including patients treated with rituximab only. It was suggested that the reason for nonresponsiveness in that patient group is B-cell depletion caused by rituximab. Additional studies with smaller patient numbers yielded similar results. None of the patients who received rituximab in the studies by Ljungman et al. [6] or Takata et al. [7] developed immunity to the influenza vaccine administered.
In a recent meta-analysis evaluating vaccine responsiveness after anti-CD20 therapy, patients on active (<3 months since last dose) anti-CD20 therapy had poor responses to all types of vaccines and abrogated responses for at least 6 months after treatment. For all vaccine types, response improved incrementally over time, but did not reach the level of healthy controls even 12 months after therapy [8].
The present study tested humoral response to Covid-19 vaccination in NHL patients treated with anti-CD20 mAbs. The results strongly suggest that most patients with low CD19 lymphocyte counts caused by treatment with anti-CD20 mAbs do not achieve a seropositive response after Covid-19 vaccination.
Tzarfati et al. [9] reported a lower serologic response in patients with hematologic malignancies after BNT162b2 vaccination (75% vs. 99%, p < 0.001). Patients treated with anti-CD20 antibodies had significantly fewer seropositive responses and lower median antibody titers.
Bonelli et al. [10] reported similar results in a study evaluating the humoral and cell-mediated response in 5 patients with rheumatologic disease. They had received their last treatment of rituximab 4–12 months before Covid-19 vaccination. Three of these patients did not have detectable CD19+ B cells and did not develop an antibody response, whereas two of the patients with detectable CD19+ B cells exhibited an antibody response. Despite diminished humoral response, interferon-γ response to SARS-Cov-2 peptides was observed in all patients, suggesting T-cell activity irrespective of the antibody response. However, there are no data yet on whether T-cell activity is sufficient to protect vaccinated patients from Covid-19 infection.
In an additional study, Bedognetti et al. [11] evaluated the humoral response to influenza vaccine in 14 NHL patients beyond the rituximab treatment period compared with healthy volunteers. Median time after rituximab was 33 months (range: 14–78), B-Cell proportions (CD19+) were similar in patients and volunteers. Even though patients had an attenuated response to influenza antigens, they did reach acceptable seroprotection rates [11].
We recognize that the ideal control cohort would consist of patients with NHL who were never treated with rituximab. However, because of the universal acceptance of monoclonal anti-CD20 therapy as standard of care in NHL, such a population is exceedingly uncommon.
Conclusions
Patients and physicians should be aware that vaccination within 6 months of treatment with anti-CD20 mAbs may not confer immunity against Covid-19. Vaccination of close contacts, adherence to hygiene, social distancing, and particularly avoidance of close contact with ill people might be needed to optimally protect this population from Covid-19. For patients expected to need anti-CD20 monoclonal antibodies in the near future, vaccination against Covid-19 before treatment should be considered. Although intuitive, additional studies are necessary to confirm whether a third vaccination is indicated in patients after treatment with anti-CD20 mAbs once peripheral B cells are repopulated.
Conflict of interest disclosure
The authors declare no competing financial interests.
References
- 1.Polack FD, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baden RL, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yri OE, Torfoss D, Hungnes O, et al. Rituximab blocks protective serologic response to influenza A (H1N1) 2009 vaccination in lymphoma patients during or within 6 months after treatment. Blood. 2011;118:6769–6771. doi: 10.1182/blood-2011-08-372649. [DOI] [PubMed] [Google Scholar]
- 4.Israel Ministry of Health. Epidemiology Department . 2021. Coronavirus (COVID-19) vaccine. Vaccine instruction. Updated 9 January. https://www.health.gov.il/UnitsOffice/HD/PH/epidemiology/td/docs/365_Corona.pdf. [Google Scholar]
- 5.Centkowski P, Brydak L, Machala M, et al. Immunogenicity of influenza vaccination in patients with non-Hodgkin lymphoma. J Clin Immunol. 2007;27:339–346. doi: 10.1007/s10875-007-9073-3. [DOI] [PubMed] [Google Scholar]
- 6.Ljungman P, Nahi H, Linde A. Vaccination of patients with haematological malignancies with one or two doses of influenza vaccine: a randomized study. Br J Haematol. 2005;130:96–98. doi: 10.1111/j.1365-2141.2005.05582.x. [DOI] [PubMed] [Google Scholar]
- 7.Takata T, Suzumiya J, Ishikawa T, et al. Attenuated antibody reaction for the primary antigen but not for the recall antigen of influenza vaccination in patients with non-Hodgkin B-cell lymphoma after the administration of rituximab–CHOP. J Clin Exp Hematol. 2009;49:9–13. doi: 10.3960/jslrt.49.9. [DOI] [PubMed] [Google Scholar]
- 8.Vijenthira A, Gong I, Betschel SD, et al. Vaccine response following anti-CD20 therapy: a systematic review meta-analysis of 905 patients. Blood Adv. 2011;5:2624–2643. doi: 10.1182/bloodadvances.2021004629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tzarfati KH, Gutwein O, Apel A, et al. BNT162b2 COVID-19 vaccine is significantly less effective in patients with hematologic malignancies. Am J Hematol. 2021;96:1195–1203. doi: 10.1002/ajh.26284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bonelli MM, Mrak D, Perkmann T, et al. SARS-CoV-2 vaccination in rituximab-treated patients: evidence for impaired humoral but inducible cellular immune response. Ann Rheum Dis. 2021;80:1355–1356. doi: 10.1136/annrheumdis-2021-220408. [DOI] [PubMed] [Google Scholar]
- 11.Bedognetti D, Ansaldi F, Zanard E, et al. Seasonal and pandemic (A/H1N1 2009) MF59-adjuvanted influenza vaccines in complete remission non-Hodgkin lymphoma patients previously treated with rituximab containing regimens. Blood. 2012;120:1954–1957. doi: 10.1182/blood-2012-06-438689. [DOI] [PMC free article] [PubMed] [Google Scholar]