Summary
Esophageal cancer is the seventh most common cancer, with an estimated 572,000 new cases, and the sixth most common cause of cancer-related deaths in 2018 with 509,000 annual worldwide deaths. Advanced esophageal squamous cell carcinoma (ESCC) is one of devastating tumors with a 5-year survival rate of less than 5% in patients with metastatic disease. Treatment options for patients with advanced ESCC are limited. Current guidelines recommend chemotherapy containing a platinum and a fluoropyrimidine agent as a first-line treatment. Recently, immune checkpoint inhibitors (ICIs), including nivolumab and pembrolizumab, have demonstrated antitumor activity and clinical efficacy in patients with advanced ESCC that is refractory or intolerant to first-line chemotherapy. ICIs are game-changers that not only transformed oncological strategy but also have a wide range of clinical potential in combination with conventional cytotoxic chemotherapy and radiotherapy. There is still an urgent, unmet need for reliable treatment options to conquer this intractable disease.
Keywords: esophageal squamous cell carcinoma, immune checkpoint inhibitor, nivolumab, pembrolizumab
Introduction
Esophageal cancer is the seventh most common cancer, with an estimated 572,000 new cases, and the sixth most common cause of cancer-related deaths in 2018, with 509,000 deaths annually worldwide (1). Esophageal cancer comprises squamous cell carcinoma (SCC), adenocarcinoma (AC), and other minor histological subtypes. SCC and AC have distinct etiological and clinicopathological features.
Esophageal SCC (ESCC) is generally in decline in North America and Europe, where alcohol drinking and smoking are the major risk factors. However, the incidence is still extremely high in parts of Asia and Sub-Saharan Africa. In contrast, the incidence of esophageal AC (EAC) is rapidly rising around high-income countries in North America and Western Europe, where increasing obesity and gastroesophageal reflux disease may explain the high prevalence (1,2).
Large-scale genomic analysis of ESCC using next-generation sequencing revealed distinct profiles of genomic alterations between ESCC and EAC. KRAS and ERBB2 were more frequently altered in EAC than in ESCC, whereas NOTCH1 was more commonly altered in ESCC than in EAC (3). Molecularly targeted therapies focusing on HER2 (trastuzumab) or vascular endothelial growth factor (ramucirumab) are applicable in patients with gastroesophageal AC (4-6). The number of non-synonymous somatic mutations in ESCC was relatively high among solid tumors except for colorectal cancer. However, the diver mutation has not been identified in ESCC (7,8).
A multidisciplinary approach involving surgery, radiotherapy, and chemotherapy is the mainstay of treatment that improves prognosis in resectable ECC. However, the 5-year survival rate is less than 5% in patients with unresectable advanced ECC, which is defined as locally advanced, metastatic, or recurrent ECC without indication for curative surgery (9). Although survival benefit over the best supportive care is not sufficiently demonstrated, fluoropyrimidine and platinum doublet chemotherapy is recommended as an acceptable first-line therapy for patients with metastatic or recurrent ESCC according to current guidelines (10-12). In Japan, combination chemotherapy of cisplatin and 5-fluorouracil (CF) is recognized as the first-line regimen based on two phase 2 trials (JCOG8807 and JCOG9407) which demonstrated a response rate of 33.3-35.9% and median overall survival (OS) of 6.7 months (13,14). The clinical efficacy of docetaxel, cisplatin and 5- fluorouracil (DCF) regimen for advanced ESCC was demonstrated with a favorable response rate of around 60% in early phase trials (15-17). The incidence of grade 3/4 neutropenia and grade 3/4 febrile neutropenia were high as 43.6- 90.0% and 10.0-22.2%, respectively. A phase 3 trial (JCOG 1314) comparing modified DCF with CF as first-line chemotherapy in patients with metastatic or recurrent esophageal cancer is ongoing.
Taxanes (docetaxel and paclitaxel) and irinotecan are commonly used agents as second-line treatment in patients with advanced ESCC if the first-line therapy fails. Docetaxel as a single agent for metastatic esophageal cancer was effective with a response rate of 16% and median OS of 8.1 months, although grade 3/4 neutropenia occurred in 88% of patients (18). Weekly paclitaxel as second-line therapy for advanced or recurrent esophageal had shown efficacy with a response rate of 44.2% and median OS of 10.4 months. The most common grade 3/4 adverse events were neutropenia which occurred in 52.8%, and adverse events leading to discontinuation of therapy occurred in 34% of patients (19). Irinotecan administered in repeated 6-week cycles for patients with unresectable esophageal carcinoma demonstrated a response rate of 22.2% and median OS of 7.5 months, while grade 3/4 neutropenia and diarrhea occurred in 5% and 5.8% of patients, respectively (20). Paclitaxel, irinotecan, or oxaliplatin-based combination regimens have also been investigated as first or second-line therapy for advanced ESCC. The combination regimens showed some efficacy; however, a relatively high incidence of toxicities is of concern given the vulnerable conditions of the patients with advanced ESCC.
Immune checkpoint inhibitors (ICIs)
Immune checkpoints are a part of the immune system that modulates the immune response to attack tumor cells. Immune checkpoint molecules are present on T cells, antigen-presenting cells (APCs), and tumor cells. The interactions among the molecules stimulate either inhibitory or activating immune signaling pathways. The inhibitory signaling pathway plays a pivotal role in inducing immune tolerance and suppressing autoimmune responses. Some cancers escape from the attack by stimulating inhibitory signals. Immune checkpoint molecules include programmed cell death protein 1 (PD- 1) and CTLA-4 (cytotoxic T-lymphocyte associated antigen 4) on T-cells and programmed death-ligand 1 (PD-L1) and B7-1/B7-2 on APCs, including tumor cells. Blocking the binding of PD-L1 to PD-1 or B7-1/B7-2 to CTLA-4 with an ICI allows the T-cells to activate and destroy the tumor cells (21,22). Immune checkpoint molecules and representative ICIs targeting inhibitory immunoreceptors are illustrated in Figure 1.
Figure 1.
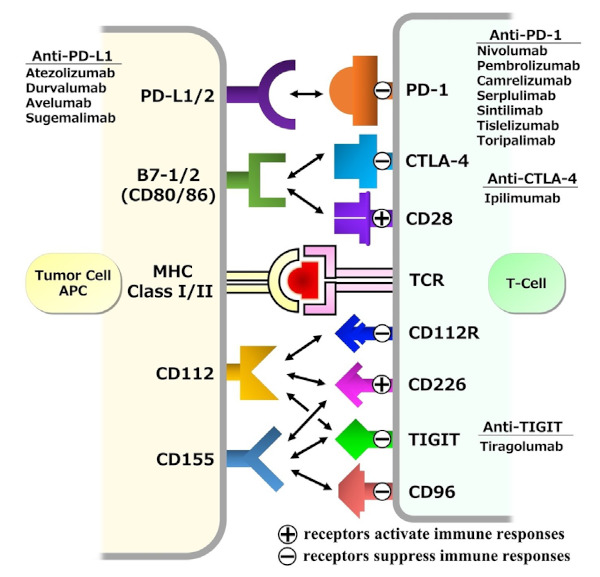
Immune checkpoint molecules and representative immune checkpoint inhibitor agents targeting inhibitory immunoreceptors. APC, antigen-presenting cell; CTLA-4, cytotoxic T-lymphocyte associated antigen 4; MHC, major histocompatibility complex; PD-1, programmed cell death protein 1; PD-L1/2, programmed cell death protein ligand 1 and 2; TCR, T-cell receptor; TIGIT, T-cell immunoreceptor with Ig and ITIM domains.
The clinical use of checkpoint inhibitors has dramatically changed the therapeutic strategy for malignant melanoma. The antitumor activity of these agents has been reported in gastrointestinal AC and SCC of the esophagus, head and neck, lung, and anus with promising efficacy (23-28). The first ICI approved by the Food and Drug Administration (FDA) was ipilimumab (anti-CTLA-4 antibody), followed by nivolumab and pembrolizumab (anti-PD-1 antibodies).
Tumor PD-L1 expression is thought to be correlated with tumor susceptibility to immune checkpoint inhibition and favorable prognosis. In patients with ESCC, the PD-L1 expression rate ranges from 15% to 83% in tumor cells, and from 13% to 31% in immune cells (29-34).
ICIs can induce unique side effects known as immune-related adverse events (irAEs). These irAEs are distinct from AEs of traditional cytotoxic agents and typically exhibit a delayed onset and prolonged duration. These irAEs are rare and generally low grade; however, some irAEs can be severe and may lead to fatal consequences. Although irAEs can affect almost any organ, frequent toxicities involve the skin, thyroid gland, lung, liver, and pituitary gland. The treatment includes temporary discontinuation of ICIs and the use of glucocorticoids as well as monoclonal antibody therapy or plasma exchange for severe cases. Monitoring the functions of some organs, such as the thyroid and liver, is recommended to detect irAE early before it becomes evident (35).
Nivolumab
Nivolumab is a human IgG4 monoclonal anti-PD-1 antibody that was approved for the treatment of melanoma by the FDA in 2014. Clinical trials revealed promising clinical efficacy with manageable adverse effects in several solid tumors, including advanced ESCC.
An open-label, multicenter, phase 2 trial (ONO-4538- 07) investigated the safety and activity of nivolumab in patients with advanced ESCC that was refractory or intolerant to fluoropyrimidine-based, platinum-based, and taxane-based chemotherapies. Seventeen percent of 64 patients had an objective response with central assessment. The median OS and progression-free survival (PFS) were 10.8 months and 1.6 months, respectively. Common adverse events included pneumonia and interstitial lung disease, all of which were manageable with treatment discontinuation or appropriate care (28).
T h e r a n d o m i z e d , o p e n - l a b e l , p h a s e 3 ATTRACTION-3 trial compared the clinical efficacy of nivolumab with that of chemotherapy in patients with unresectable advanced or recurrent ESCC that was refractory or intolerant to one previous line of fluoropyrimidine-based and platinum-based chemotherapy. The OS was significantly improved in the nivolumab group compared with the chemotherapy group (median 10.9 months vs. 8.4 months; HR for death 0.77, 95% CI 0.62-0.96, p = 0.019). PD-L1 expression in tumors was not correlated with the survival benefit with nivolumab, although patients with at least 1% PD-L1 expression had a 15% reduction in the risk of death than those with less than 1% PD-L1 expression. Despite the favorable OS in the nivolumab group, treatment with nivolumab did not provide a significant benefit in PFS over chemotherapy (median 1.7 months vs. 3.4 months; HR for progression or death 1.08, 95% CI 0.87-1.34). Common treatment-related adverse events included rash (11%), diarrhea (12%), and decreased appetite (11%) in the nivolumab group, whereas alopecia (47%), neutropenia (37%), and leukocytopenia (35%) were observed in the chemotherapy group. Serious adverse events and treatment-related deaths occurred in 16% of nivolumab patients and 23% of chemotherapy patients. Treatment-related deaths were reported in 1% of patients treated with both nivolumab and chemotherapy (36).
Pembrolizumab
Pembrolizumab is a selective human IgG4 monoclonal antibody designed to inhibit the interaction between PD-1 and its ligands, PD-L1 and PD-L2. The FDA approved pembrolizumab for clinical use in recurrent or metastatic head and neck SCC in June 2018, based on the KEYNOTE-012 study, a phase 1B trial that evaluated the safety and antitumoral activity of pembrolizumab in patients with head and neck cancer with any level of PD-L1 expression. The proportion of patients with an overall response was 18% (8 of 45 patients; 95% CI 8-32). Twenty-three out of 28 patients had maintained tumor shrinkage over six months (37).
KEYNOTE-028, a phase IB study, investigated the safety and antitumor activity of pembrolizumab in patients with PD-L1 positive, advanced solid tumors. Twenty-three patients with PD-L1 positive esophageal or gastroesophageal junction carcinoma received pembrolizumab after standard therapy failed. Overall responses were observed in 29.4% of patients with SCC and 40.0% of patients with AC. Twelve patients (52%) showed tumor shrinkage from baseline in target lesion burden. Median overall survival was 7.0 months, and the 12-month overall survival rates were 40% (38).
The phase 2 KEYNOTE-180 study evaluated the efficacy and safety of pembrolizumab for patients with advanced ESCC, EAC, or esophagogastric junction AC after two or more lines of systemic therapy. The response rate was 9.9% among 63 patients with ESCC, 13.8% among 58 patients with PD-L1-positive tumors, and 6.3% among 63 patients with PD-L1-negative tumors. The trial confirmed durable antitumor activity and manageable safety (39).
The randomized, open-label, global, phase 3 study, KEYNOTE-181, enrolled 628 patients with metastatic, locally advanced unresectable ESCC, EAC, or esophagogastric junction AC that progressed after one prior line of standard therapy (40). The patients were randomly assigned 1:1 to pembrolizumab or the investigator's choice of standard-of-care chemotherapy with paclitaxel, docetaxel, or irinotecan. A PD-L1- positive tumor was defined as the combined positive score (CPS) of 10 or more, as previously described in phase 2 KEYNOTE-180 study. Pembrolizumab provided a significantly better OS compared with chemotherapy in patients with metastatic ESCC and PD-L1 CPS ≥ 10 (Table 1). On retrospective stratified analysis, OS was remarkably improved in the pembrolizumab group compared with the chemotherapy group (median 10.3 months vs. 6.7 months; HR for death 0.64, 95% CI 0.46-0.90) among SCC patients with PD-L1 CPS ≥ 10. In patients with PD-L1 CPS ≥ 10, PFS was also improved in the pembrolizumab group compared with the chemotherapy group (median 2.6 months vs. 3.0 months; HR 0.73, 95% CI 0.54- 0.97). Treatment-related adverse events were observed in 64% of patients in the pembrolizumab group and 86% of patients in the chemotherapy group. The common adverse events in the pembrolizumab group included fatigue (11.8%), hypothyroidism (10.5%), decreased appetite (8.6%), asthenia (7.0%), and nausea (7.0%). Immune-mediated adverse events and infusion reactions occurred in 23.2% of patients, comparable with results observed in previous studies (28,36,39).
Table 1. Results of the stratified analysis in the randomized phase 3 KEYNOTE-181 study.
| Treatment | Patients | Median OS [95% CI] (months) | HR | p value | |
|---|---|---|---|---|---|
| All | Pembrolizumab | 314 | 7.1 [6.2-8.1] | 0.89 [0.75-1.05] | 0.08431 |
| Chemotherapy | 314 | 7.1 [6.3-8.0] | |||
| CPS ≥ 10 | Pembrolizumab | 107 | 9.3 [6.6-12.5] | 0.70 [0.52-0.94] | 0.00855 |
| Chemotherapy | 115 | 6.7 [5.1-8.2] | |||
| SCC | Pembrolizumab | 198 | 8.2 [6.7-10.3] | 0.77 [0.63-0.96] | 0.00894 |
| Chemotherapy | 203 | 7.1 [6.1-8.2] |
CI, confidence interval; CPS, combined positive score; HR, hazard ratio; OS, overall survival; SCC, squamous cell carcinoma.
Based on the results of the reliable phase 3 clinical trials, the Japan Esophageal Society issued a public statement recommending treatment with nivolumab as second-line therapy in patients with metastatic, locally advanced unresectable, and recurrent esophageal cancer irrespective of the PD-L1 expression status, and treatment with pembrolizumab as second-line therapy in patients with metastatic, locally advanced unresectable ESCC with PD-L1 CPS ≥ 10. Results from the pivotal clinical trials that demonstrated the antitumor activity and clinical efficacy of nivolumab and pembrolizumab are summarized (Table 2).
Table 2. Results from a selected clinical trial using immune checkpoint inhibitors in advanced esophageal squamous cell carcinoma.
| Agents (Trial) |
Phase | Line | Location | Histology | Enrolled Patients | Regimen | Response Rate | Median PFS | Median OS |
|---|---|---|---|---|---|---|---|---|---|
| Nivolumab (ONO-4583-07) | 2 | ≥ 2 | E | SCC | 65 | Nivolumab | 17% | 1.6M | 10.8M |
| Nivolumab (ATTRACTION-3) | 3 | ≥ 2 | E/EGJ | SCC/AC | 419 | Nivolumab vs. PTX or DTX | 19% vs. 22% | 1.7M vs. 3.4M | 10.9M vs. 8.4M |
| Pembrolizumab (KEYNOTE-028) | 1 | ≥ 2 | E/EGJ | (PD-L1+) | 23 | Pembrolizumab | All: 30% SCC: 28% AC: 40% |
1.8M | 7.0M |
| Pembrolizumab (KEYNOTE-180) | 2 | ≥ 3 | E/EGJ | SCC/AC | 121 | Pembrolizumab | All: 9.9% SCC: 14.3% AC: 5.2% PD-L1(+): 13.8% PD-L1(-): 6.3% |
All: 2.0M SCC: 2.1M AC: 1.9M PD-L1(+): 2.0M PD-L1(-): 2.0M |
All: 5.8M SCC: 6.8M AC: 3.9M PD-L1(+): 6.3M PD-L1(-): 5.4M |
| Pembrolizumab (KEYNOTE-181) | 3 | 2 | E/EGJ | SCC/AC | 628 | Pembrolizumab vs. PTX or DTX or CPT-11 | All: 13.1% vs 6.7% PD-L1(+): 21.5% vs. 6.1% SCC: 16.7% vs. 7.4% |
All: 2.1M vs. 3.4M PD-L1(+): 2.6M vs. 3.0M SCC: 2.2M vs. 3.1M |
All: 7.1M vs. 7.1M PD-L1(+): 9.3M vs. 6.7M SCC: 8.2M vs. 7.1M |
| Camrelizumab (ESCORT) | 3 | 2 | E | SCC | 457 | Camrelizumab vs. DTX or CPT-11 | 20.2% vs. 6.4% | 1.9M vs. 1.9M | 8.3M vs. 6.2M |
AC, adenocarcinoma; CPS, combined positive score; CPT-11, irinotecan; DTX. docetaxel; E, esophagus; EGJ, esophagogastric junction; HR, hazard ratio; M, months; OS, overall survival; PD-L1, programmed cell death protein ligand 1; PFS, progression-free survival; PTX, paclitaxel; SCC: squamous cell carcinoma.
Camrelizumab is another humanized, selective IgG4 monoclonal antibody against PD-1. The antitumoral activity was proved across a variety of solid tumors (41,42). A randomized, open-label, phase 3 study, ESCORT trial was conducted in 43 hospitals in China. Six hundred seven patients with advanced, recurrent, or metastatic ESCC were randomly assigned to camrelizumab or chemotherapy (docetaxel or irinotecan) as second-line treatment. The result showed a favorable median OS of 8.3 months (95% CI 6.8-9.7) in the camrelizumab group, as compared to 6.2 months (5.7-6.9) in the chemotherapy group (hazard ratio 0.71 [95% CI 0.57-0.87], p = 0.001) (43).
Combination of immune checkpoint inhibitors and radiation therapy or cytotoxic agents
The standard treatment for unresectable locally advanced ESCC is chemoradiotherapy (CRT) (44). The potential synergy of combination therapy with ICIs and radiation is attracting attention lately. The abscopal effect signifies the regression of non-irradiated lesions located distally from the primary site of irradiation. A persuasive theory explains a systemic antitumor immune response may mediate the effect (45,46). The mechanism of the effect may include the recruitment and distribution to the microenvironment of T-cells, cytokine release, and enhanced presentation of tumor antigen. Preclinical studies indicated increased PD-L1 expression in irradiated tumors, which prompts the need for enhanced antitumor activity by combined therapy with anti-PD-1 antibodies and radiation in locally advanced ESCC (47). Furthermore, radiation can potentially promote the antigen presentation of tumors. This is one of the rationales that support the validity of combination therapy of radiation and ICIs (48).
A phase 3 PACIFIC trial compared durvalumab (anti- PD-L1 antibody agent) following platinum-based CRT with placebo following CRT for unresectable locally advanced non-small-cell lung cancer. Twelve months of durvalumab after CRT significantly prolonged both PFS (16.8 vs. 5.6 months; HR: 0.52; 95% CI: 0.42-0.65, p < 0.001) and OS (not reached vs. 28.7 months, HR: 0.68; 99.73% CI: 0.47-0.997; p = 0.0025) compared with acceptable radiation or ICI-induced pneumonitis (49).
Phase 3 clinical trials investigating the efficacy and safety of the combination of ICIs with chemoradiation therapy for locally advanced ESCC are in progress (NCT04543617, NCT04550260).
A phase 3 randomized, international, double-blind study KEYNOTE-590 (NCT03189719) was conducted to compare pembrolizumab plus chemotherapy (CF regimen) with chemotherapy alone in patients with locally advanced, unresectable, or metastatic EAC, ESCC, or Siewert type 1 gastroesophageal junction adenocarcinoma. 749 patients were enrolled, and the OS superiority of pembrolizumab plus chemotherapy was demonstrated in patients with ESCC (median 12.6 vs. 9.8 months; HR 0.72; 95% CI, 0.60-0.88; p = 0.0006), CPS ≥ 10 (median 13.5 vs. 9.4 months; HR 0.62; 95% CI, 0.49-0.78; p < 0.0001), and all patients (median 12.4 vs. 9.8 months; HR, 0.73; 95% CI, 0.62-0.86; p < 0.0001). Addition of pembrolizumab also provided longer PFS (median 6.3 vs. 5.8 months; HR 0.65; 95% CI, 0.55-0.76; p < 0.0001) and better response rate (45.0% vs. 29.3%; p < 0.0001), with acceptable safety profile (50). Based on the result, the FDA has approved pembrolizumab for use in combination with platinum and fluoropyrimidine-based chemotherapy for patients with metastatic or locally advanced esophageal or gastroesophageal carcinoma in March 2021.
Dual immune checkpoint inhibition therapy to enhance the efficacy of immunotherapy has also been investigated. The CheckMate 648 (NCT03143153) study is a randomized phase 3 trial to compare the efficacy of nivolumab plus CF vs. nivolumab plus ipilimumab vs. CF alone as first-line therapy in patients with unresectable advanced, recurrent, or metastatic ESCC. Both nivolumab plus chemotherapy and nivolumab plus ipilimumab demonstrated superior OS over chemotherapy (median 15.4 vs. 13.7 vs. 9.1 months) in patients with tumor cell PD-L1 ≥ 1%. The OS superiority was also confirmed in all patients with both nivolumab plus chemotherapy and nivolumab plus ipilimumab compared with chemotherapy alone: 13.2, 12.8, and 10.7 months, respectively (51).
Many phase 3 trials evaluating the efficacy of a wide range of combinations of ICIs with cytotoxic agents and radiation therapy are now in progress worldwide (Table 3). The results of these studies will dramatically change the practical strategy for the treatment of advanced ESCC furthermore.
Table 3. List of ongoing randomized phase 3 clinical trials for advanced esophageal cancer.
| NCT number (Title of the trial) |
Target | Agent | Line | Location | Histology | Eligible Condition | Treatment Arm | Enrolled Patients | Primary Outcome |
|---|---|---|---|---|---|---|---|---|---|
|
NCT04543617 (SKYSCRAPER 07) |
PD-L1 TIGIT |
Atezolizumab Tiragolumab |
1 | E | SCC | LA | Tiragolumab + Atezolizumab + dCRT placebo + Atezolizumab + dCRT placebo + dCRT |
750 | OS/PFS |
|
NCT04540211 (SKYSCRAPER 08) |
PD-L1 TIGIT |
Atezolizumab Tiragolumab |
1 | E | SCC/AC | LA, M, R | Atezolizumab + Tiragolumab + PC placebo + PC |
450 | OS/PFS |
| NCT04426955 | PD-1 | Camrelizumab | 1 | E | SCC | LA | Camrelizumab + PC + RT placebo + PC + RT |
390 | PFS |
| NCT03691090 | PD-1 | Camrelizumab | 1 | E | SCC/AC | LA, M, R | Camrelizumab + PC placebo + PC |
548 | OS/PFS |
| NCT04550260 | PD-L1 | Durvalumab | 1 | E | SCC | LA | Durvalumab + dCRT placebo + dCRT |
600 | PFS |
|
NCT04210115 (KEYNOTE 975) |
PD-1 | Pembrolizumab | 1 | E/GEJ | SCC/AC | LA | Pembrolizumab + FP or FOLFOX + RT placebo + FP or FOLFOX + RT |
600 | OS/EFS |
|
NCT03189719 (KEYNOTE 590) |
PD-1 | Pembrolizumab | 1 | E/GEJ | SCC/AC | LA, M | Pembrolizumab + FP placebo + FP |
749 | OS/PFS |
| NCT03958890 | PD-1 | Serplulimab | 1 | E | SCC | LA, M, R | Serplulimab + FP placebo + FP |
489 | PFS |
|
NCT03748134 (ORIENT 15) |
PD-1 | Sintilimab | 1 | E | SCC | LA, M, R | Sintilimab + FP or PC placebo + FP or PC |
676 | OS |
| NCT04187352 | PD-L1 | Sugemalimab | 1 | E | SCC | LA, M, R | Sugemalimab + FP placebo + FP |
420 | OS/PFS |
| NCT03957590 | PD-1 | Tislelizumab | 1 | E | SCC | LA | Tislelizumab + PC + RT placebo + PC + RT |
316 | PFS |
| NCT03783442 | PD-1 | Tislelizumab | 1 | E | SCC | LA, M, R | Tislelizumab + chemotherapy placebo + chemotherapy |
649 | OS/PFS |
|
NCT03829969 (JUPITER 06) |
PD-1 | Toripalimab | 1 | E | SCC | LA, M, R | Toripalimab + PC placebo + PC |
500 | OS/PFS |
|
NCT03143153 (CheckMate 648) |
PD-1 CTLA-4 |
Nivolumab Ipilimumab |
1 | E | SCC/AC | LA, M, R | Nivolumab + Ipilimumab Nivolumab + FP FP |
970 | OS/PFS |
| NCT03430843 | PD-1 | Tislelizumab | 2 | E | SCC | LA, M | Tislelizumab Paclitaxel or Docetaxel or Irinotecan |
513 | OS |
AC, adenocarcinoma; Adj, adjuvant chemotherapy; CTLA-4, cytotoxic T-lymphocyte associated antigen 4; dCRT, definitive chemoradiotherapy; DFS, disease-free survival; E, esophagus; EFS, event-free survival; FP, 5-fluorouracil + cisplatin; GEJ, gastroesophageal junction; LA, locally advanced disease; M, metastatic disease; OS, overall survival; PC, paclitaxel and cisplatin; PD-1, programmed cell death protein 1; PD-L1, programmed cell death protein ligand 1; PFS, progression-free survival; R, recurrent disease; RT, radiotherapy; SCC, squamous cell carcinoma; TIGIT, T cell immunoreceptor with Ig and ITIM domains
Conclusion
Based on the results of randomized, phase 3 trials, nivolumab and pembrolizumab were approved by the Japanese Ministry of Health, Labor and Welfare as second-line therapy for patients with unresectable advanced esophageal cancer in February and August 2020, respectively. The approval of novel agents against esophageal cancer was granted for the first time in eight years in Japan. ICIs transformed oncological strategy and have a wide range of clinical potential in combination with conventional cytotoxic chemotherapy and radiotherapy.
Funding: This work was supported by the National Center for Global Health and Medicine.
Conflict of Interest
The authors have no conflicts of interest to disclose.
References
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018; 68:394-424. [DOI] [PubMed] [Google Scholar]
- 2. Lin Y, Totsuka Y, He Y, Kikuchi S, Qiao Y, Ueda J, Wei W, Inoue M, Tanaka H. Epidemiology of esophageal cancer in Japan and China. J Epidemiol. 2013; 23:233-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wang K, Johnson A, Ali SM, Klempner SJ, Bekaii- Saab T, Vacirca JL, Khaira D, Yelensky R, Chmielecki J, Elvin JA, Lipson D, Miller VA, Stephens PJ, Ross JS. Comprehensive genomic profiling of advanced esophageal squamous cell carcinomas and esophageal adenocarcinomas reveals similarities and differences. Oncologist. 2015; 20:1132-1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fuchs CS, Tomasek J, Yong CJ, et al. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): An international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet. 2014; 383:31-39. [DOI] [PubMed] [Google Scholar]
- 5. Wilke H, Muro K, Van Cutsem E, et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): A double-blind, randomised phase 3 trial. Lancet Oncol. 2014; 15:1224-1235. [DOI] [PubMed] [Google Scholar]
- 6. Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): A phase 3, open-label, randomised controlled trial. Lancet. 2010; 376:687-697. [DOI] [PubMed] [Google Scholar]
- 7. Vogelstein B, Papadopoulos N, Velculescu VE, Zhou S, Diaz LA Jr, Kinzler KW. Cancer genome landscapes. Science. 2013; 339:1546-1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lin DC, Hao JJ, Nagata Y, et al. Genomic and molecular characterization of esophageal squamous cell carcinoma. Nat Genet. 2014; 46:467-473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Noone AM, Cronin KA, Altekruse SF, Howlader N, Lewis DR, Petkov VI, Penberthy L. Cancer incidence and survival trends by subtype using data from the surveillance epidemiology and end results program, 1992-2013. Cancer Epidemiol Biomarkers Prev. 2017; 26:632-641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ajani JA, D'Amico TA, Bentrem DJ, et al. Esophageal and esophagogastric junction cancers, Version 2.2019, NCCN Clinical Practice Guidelines in Oncology J Natl Compr Canc Netw. 2019; 17:855-883 [DOI] [PubMed] [Google Scholar]
- 11. Kitagawa Y, Uno T, Oyama T, et al. Esophageal cancer practice guidelines 2017 edited by the Japan Esophageal Society: part 1. Esophagus. 2019; 16:1-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Muro K, Lordick F, Tsushima T, et al. Pan-Asian adapted ESMO Clinical Practice Guidelines for the management of patients with metastatic oesophageal cancer: A JSMO-ESMO initiative endorsed by CSCO, KSMO, MOS, SSO and TOS. Ann Oncol. 2019; 30:34-43. [DOI] [PubMed] [Google Scholar]
- 13. Iizuka T, Kakegawa T, Ide H, et al. Phase II evaluation of cisplatin and 5-fluorouracil in advanced squamous cell carcinoma of the esophagus: a Japanese Esophageal Oncology Group Trial. Jpn J Clin Oncol. 1992; 22:172-176. [PubMed] [Google Scholar]
- 14. Hayashi K, Ando N, Watanabe H, et al. Phase II evaluation of protracted infusion of cisplatin and 5-fluorouracil in advanced squamous cell carcinoma of the esophagus: a Japan Esophageal Oncology Group (JEOG) Trial (JCOG9407). Jpn J Clin Oncol. 2001; 31:419-423. [DOI] [PubMed] [Google Scholar]
- 15. Takahashi H, Arimura Y, Yamashita K, et al. Phase I/ II study of docetaxel/cisplatin/fluorouracil combination chemotherapy against metastatic esophageal squamous cell carcinoma. J Thorac Oncol. 2010; 5:122-128. [DOI] [PubMed] [Google Scholar]
- 16. Yamasaki M, Miyata H, Tanaka K, et al. Multicenter phase I/II study of docetaxel, cisplatin and fluorouracil combination chemotherapy in patients with advanced or recurrent squamous cell carcinoma of the esophagus. Oncology. 2011; 80:307-313. [DOI] [PubMed] [Google Scholar]
- 17. Tamura S, Imano M, Takiuchi H, et al. Phase II study of docetaxel, cisplatin and 5-fluorouracil (DCF) for metastatic esophageal cancer (OGSG 0403). Anticancer Res. 2012; 32:1403-1408. [PubMed] [Google Scholar]
- 18. Muro K, Hamaguchi T, Ohtsu A, et al. A phase II study of single-agent docetaxel in patients with metastatic esophageal cancer. Ann Oncol. 2004; 15:955-959. [DOI] [PubMed] [Google Scholar]
- 19. Kato K, Tahara M, Hironaka S, et al. A phase II study of paclitaxel by weekly 1-h infusion for advanced or recurrent esophageal cancer in patients who had previously received platinum-based chemotherapy. Cancer Chemother Pharmacol. 2011; 67:1265-1272. [DOI] [PubMed] [Google Scholar]
- 20. Mühr-Wilkenshoff F, Hinkelbein W, Ohnesorge I, et al. A pilot study of irinotecan (CPT-11) as single-agent therapy in patients with locally advanced or metastatic esophageal carcinoma. Int J Colorectal Dis. 2003; 18:330-334. [DOI] [PubMed] [Google Scholar]
- 21. Brahmer JR, Drake CG, Wollner I, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: Safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 2010; 28:3167-3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang C, Thudium KB, Han M, et a l. In vitro characterization of the anti-PD-1 antibody nivolumab, BMS-936558, and in vivo toxicology in non-human primates. Cancer Immunol Res. 2014; 2:846-856. [DOI] [PubMed] [Google Scholar]
- 23. Kang YK, Boku N, Satoh T, et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017; 390:2461-2471. [DOI] [PubMed] [Google Scholar]
- 24. Moehler M, Delic M, Goepfert K, et al. Immunotherapy in gastrointestinal cancer: Recent results, current studies and future perspectives. Eur J Cancer. 2016; 59:160-170. [DOI] [PubMed] [Google Scholar]
- 25. Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015; 373:123-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ferris RL, Blumenschein G Jr, Fayette J, et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med. 2016; 375:1856-1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Morris VK, Salem ME, Nimeiri H, et al. Nivolumab for previously treated unresectable metastatic anal cancer (NCI9673): a multicentre, single-arm, phase 2 study. Lancet Oncol. 2017; 18:446-453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kudo T, Hamamoto Y, Kato K, et al. Nivolumab treatment for oesophageal squamous-cell carcinoma: an open-label, multicentre, phase 2 trial. Lancet Oncol. 2017; 18:631-639. [DOI] [PubMed] [Google Scholar]
- 29. Chen L, Deng H, Lu M, Xu B, Wang Q, Jiang J, Wu C. B7-H1 expression associates with tumor invasion and predicts patient's survival in human esophageal cancer. Int J Clin Exp Pathol. 2014; 7:6015-6023. [PMC free article] [PubMed] [Google Scholar]
- 30. Hatogai K, Kitano S, Fujii S, Kojima T, Daiko H, Nomura S, Yoshino T, Ohtsu A, Takiguchi Y, Doi T, Ochiai A. Comprehensive immunohistochemical analysis of tumor microenvironment immune status in esophageal squamous cell carcinoma. Oncotarget. 2016; 7:47252-47264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chen K, Cheng G, Zhang F, Zhang N, Li D, Jin J, Wu J, Ying L, Mao W, Su D. Prognostic significance of programmed death-1 and programmed death-ligand 1 expression in patients with esophageal squamous cell carcinoma. Oncotarget. 2016; 7:30772-30780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Salem ME, Puccini A, Xiu J, Raghavan D, Lenz HJ, Korn WM, Shields AF, Philip PA, Marshall JL, Goldberg RM. Comparative molecular analyses of esophageal squamous cell carcinoma, esophageal adenocarcinoma, and gastric adenocarcinoma. Oncologist. 2018; 23:1319-1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jiang Y, Lo AWI, Wong A, Chen W, Wang Y, Lin L, Xu J. Prognostic significance of tumor-infiltrating immune cells and PD-L1 expression in esophageal squamous cell carcinoma. Oncotarget. 2017; 8:30175-30189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Qu HX, Zhao LP, Zhan SH, Geng CX, Xu L, Xin YN, Jiang XJ. Clinicopathological and prognostic significance of programmed cell death ligand 1 (PD-L1) expression in patients with esophageal squamous cell carcinoma: A meta-analysis. J Thorac Dis. 2016; 8:3197-3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ramos-Casals M, Brahmer JR, Callahan MK, et al. Immune-related adverse events of checkpoint inhibitors. Nat Rev Dis Primers. 2020; 6:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kato K, Cho BC, Takahashi M, et al. Nivolumab versus chemotherapy in patients with advanced oesophageal squamous cell carcinoma refractory or intolerant to previous chemotherapy (ATTRACTION-3): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019; 20:1506-1517. [DOI] [PubMed] [Google Scholar]
- 37. Seiwert TY, Burtness B, Mehra R, Weiss J, Berger R, Eder JP, Heath K, McClanahan T, Lunceford J, Gause C, Cheng JD, Chow LQ. Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-012): an open-label, multicentre, phase 1b trial. Lancet Oncol. 2016; 17:956-965. [DOI] [PubMed] [Google Scholar]
- 38. Doi T, Piha-Paul SA, Jalal SI, Saraf S, Lunceford J, Koshiji M, Bennouna J. Safety and antitumor activity of the anti-programmed death-1 antibody pembrolizumab in patients with advanced esophageal carcinoma. J Clin Oncol. 2018; 36:61-67. [DOI] [PubMed] [Google Scholar]
- 39. Shah MA, Kojima T, Hochhauser D, et al. Efficacy and safety of pembrolizumab for heavily pretreated patients with advanced, metastatic adenocarcinoma or squamous cell carcinoma of the esophagus: The phase 2 KEYNOTE-180 study. JAMA Oncol. 2019; 5:546-550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kojima T, Shah MA, Muro K, et al. Randomized phase III KEYNOTE-181 study of pembrolizumab versus chemotherapy in advanced esophageal cancer. J Clin Oncol. 2020; 38:4138-4148. [DOI] [PubMed] [Google Scholar]
- 41. Huang J, Mo H, Zhang W, Chen X, Qu D, Wang X, Wu D, Wang X, Lan B, Yang B, Wang P, Zhang B, Yang Q, Jiao Y, Xu B. Promising efficacy of SHR-1210, a novel anti-programmed cell death 1 antibody, in patients with advanced gastric and gastroesophageal junction cancer in China. Cancer. 2019; 125:742-749. [DOI] [PubMed] [Google Scholar]
- 42. Fang W, Yang Y, Ma Y, et al. Camrelizumab (SHR-1210) alone or in combination with gemcitabine plus cisplatin for nasopharyngeal carcinoma: results from two single-arm, phase 1 trials. Lancet Oncol. 2018; 19:1338-1350. [DOI] [PubMed] [Google Scholar]
- 43. Huang J, Xu J, Chen Y, et al. Camrelizumab versus investigator's choice of chemotherapy as second-line therapy for advanced or metastatic oesophageal squamous cell carcinoma (ESCORT): a multicentre, randomised, open-label, phase 3 study. Lancet Oncol. 2020; 21:832-842. [DOI] [PubMed] [Google Scholar]
- 44. Ishida K, Ando N, Yamamoto S, Ide H, Shinoda M. Phase II study of cisplatin and 5-fluorouracil with concurrent radiotherapy in advanced squamous cell carcinoma of the esophagus: a Japan Esophageal Oncology Group (JEOG)/ Japan Clinical Oncology Group trial (JCOG9516). Jpn J Clin Oncol. 2004; 34:615-619. [DOI] [PubMed] [Google Scholar]
- 45. Salama AK, Postow MA, Salama JK. Irradiation and immunotherapy: From concept to the clinic. Cancer. 2016; 122:1659-1671. [DOI] [PubMed] [Google Scholar]
- 46. Park B, Yee C, Lee KM. The effect of radiation on the immune response to cancers. Int J Mol Sci. 2014; 15:927-943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Deng L, Liang H, Burnette B, Beckett M, Darga T, Weichselbaum RR, Fu YX. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest. 2014; 124:687-695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tang C, Wang X, Soh H, et al. Combining radiation and immunotherapy: a new systemic therapy for solid tumors? Cancer Immunol Res. 2014; 2:831-838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Antonia SJ, Villegas A, Daniel D, et al. Overall Survival with Durvalumab after Chemoradiotherapy in Stage III NSCLC. N Engl J Med. 2018; 379:2342-2350. [DOI] [PubMed] [Google Scholar]
- 50. Kato K, Sun J-M, Shah MA, et al. Pembrolizumab plus chemotherapy versus chemotherapy as first-line therapy in patients with advanced esophageal cancer: The phase 3 KEYNOTE-590 study. Ann Oncol; 2020; 31:S1192-3. [Google Scholar]
- 51. Chau I, Doki Y, Ajani JA, et al. Nivolumab (NIVO) plus ipilimumab (IPI) or NIVO plus chemotherapy (chemo) versus chemo as first-line (1L) treatment for advanced esophageal squamous cell carcinoma (ESCC): First results of the CheckMate 648 study. J Clin Oncol. 2021; 39:S15; abstr LBA4001. [Google Scholar]


