Abstract
Background
A flash glucose monitoring system (FGMS; FreeStyle Libre) is useful for monitoring hypoglycemic dogs with diabetes.
Objective
To assess the utility of this FGMS in dogs with induced hypoglycemia and rapid fluctuations in blood glucose (BG) concentrations.
Animals
Twenty‐four apparently healthy research (n = 10) and teaching (n = 14) dogs.
Methods
Prospective, observational study performed in tandem with a teaching laboratory. Regular insulin was administered to dogs and resulting hypoglycemia was corrected. Before insulin administration and every 10 minutes over a 90‐minute period, serial measurements of interstitial glucose (IG) with FGMS and BG with a portable blood glucose meter (PBGM) and clinical chemistry analyzer concentrations were made. Portable blood glucose meter and FGMS readings were compared to that of the clinical chemistry analyzer. Analytical and clinical accuracy were assessed using ISO 15197:2013 criteria, including Parkes error grid analysis.
Results
The proportions of readings in the low BG range (BG <100 mg/dL) for which the test method measurement was within ±15 mg/dL of the reference BG for the PBGM and FGMS were 81.7% (161/197) and 39.1% (72/184), respectively. The proportions of readings for the PBGM and FGMS, which were not likely to affect clinical outcome according to Parkes error grid analysis, were 97.9% (233/238) and 80.1% (177/221), respectively.
Conclusions and Clinical Importance
In this model, there was limited agreement between the FGMS and reference standard BG measurements. The FGMS (measuring IG concentrations) was compared to peripheral BG concentrations, not brain‐tissue glucose concentrations, and failed to reliably detect hypoglycemia.
Keywords: dog, FreeStyle Libre, hypoglycemia, interstitial glucose
Abbreviations
- BG
blood glucose
- DKA
diabetic ketoacidosis
- DM
diabetes mellitus
- EGA
error grid analysis
- FGMS
flash glucose monitoring system
- IG
interstitial glucose
- ISO
International Organization for Standardization
- MAD
mean absolute difference
- MARD
mean absolute relative difference
- mARD
median absolute relative difference
- MRD
mean relative difference
- PBGM
portable blood glucose meter
1. INTRODUCTION
Point of care, serial glucose monitoring is important for animals with disruption of glucose homeostasis. Dogs with diabetes mellitus (DM) sometimes require glucose monitoring, whether at home or in‐hospital, for a clinician to make decisions regarding changes to their dose or type of insulin. Similarly, dogs with critical illnesses and hyperglycemia, such as diabetic ketoacidosis (DKA) or hypoglycemia, require close monitoring of blood glucose (BG) concentrations. A method that is fast, inexpensive, and that minimizes dog stress and the need for venipuncture is ideal for these situations.
Portable blood glucose meters (PBGMs) provide clinicians and owners a means of assessing BG concentrations in a timely manner, and require small sample volume. The PBGMs can use capillary or venous blood and owners using them at home can generate multiple data points over time. Several PBGMs are marketed specifically for animals and have been compared to the hexokinase reference method used by laboratory analyzers. 1 A validated PBGM (AlphaTRAK 2, Zoetis Animal Health, Parsippany, New Jersey), calibrated for dogs and cats, is widely used for rapid BG measurements using a small amount of venous or capillary whole blood. 2 , 3 Some animals might not tolerate the repeated blood sampling required to monitor BG trends without undue stress.
A flash glucose monitoring system (FGMS; FreeStyle Libre 14 day system, Abbott, Chicago, Illinois) was developed for humans with DM to minimize the number of finger pricks needed to collect capillary blood. It measures interstitial glucose (IG) concentration every minute and the readings are automatically stored in 15‐minute intervals; additionally, a reading is captured when the sensor is scanned. The sensor must be scanned at least every 8 hours to save all the IG measurements for that period of time. A continuous glucose trace can be obtained if the sensor is scanned at least once every 8 hours. As per the FreeStyle Libre product web site, this 14‐day FGMS is accurate between BG concentrations of 40 and 500 mg/dL. 4 If the IG concentration is below 40 mg/dL, “LO” will appear on the reader; if the IG concentration is above 500 mg/dL, “HI” will appear on the reader. The sensor can remain in place for up to 14 days. This monitor allows for acquisition of more data over a longer period of time as compared to traditional BG curves, while minimizing the stress of blood sampling and animal handling. Various IG monitoring systems are useful in the management of diabetic dogs. 5 , 6 , 7 , 8
Glucose diffuses from blood into the interstitial space; therefore, interstitial and BG concentrations should be correlated in most instances. 9 However, because it takes time for glucose to diffuse from the intravascular to the interstitial space, changes in IG lag behind changes in BG. The estimated physiologic lag time in dogs is 5 to 12 minutes. 10 However, the relationship between glucose dynamics in blood and the interstitial space is not linear. 11 This complex relationship raises questions about the accuracy of IG measurement in the prediction of BG during rapid changes in BG.
There is evidence that the aforementioned FGMS is useful in a clinical setting for monitoring diabetic dogs with hyperglycemia or euglycemia. 5 , 12 There is evidence that this FGMS is useful in a clinical setting for monitoring diabetic dogs with hypoglycema or euglycemia; although there is a low correlation coefficient (r = 0.43) between BG and IG for diabetic dogs with hypoglycemia (BG of <70 mg/dL). 5 The aim of this study was to assess the analytical and clinical accuracy of this FGMS in dogs with induced hypoglycemia and rapid fluctuations in BG concentrations after insulin administration according to ISO 15197:2013 criteria. 13
2. MATERIALS AND METHODS
2.1. Animals
Data were collected during a student teaching laboratory. Twenty‐four mixed breed dogs aged 1.1 to 8.4 years (median, 3.69 years) and weighing 20.8 to 35.0 kg (median, 24.5 kg) were included in the study. The dogs were members of a research colony (n = 10) or teaching colony (n = 14). The body condition score (BCS) was measured on a 9‐point scale for the 24 dogs ranged from 5 to 7 (median, 5). All of the dogs measured either 5 or 6 and 1 dog (intact female) measured 7 on the BCS scale. Twelve dogs were females (2 intact, 10 spayed) and 12 were males (1 intact, 11 neutered).
2.2. Data collection
Food was withheld from the dogs for 16 hours before data collection; water was not withheld. One hour before data collection, an FGMS sensor was placed on each dog. Skin between the scapulae was clipped and cleaned with isopropyl alcohol. In addition to the adhesive present on the sensor, 2 to 4 drops of tissue adhesive (Vetbond, 3M Animal Care Products, St. Paul, Minnesota) were added for additional adhesion. Per manufacturer guidelines, the sensor was allowed 1 hour to acclimate before any readings were obtained. Each dog had its own FGMS sensor and reader, as well as its own AlphaTRAK PBGM, for the duration of the experiment. A 22G x 1‐1/4″ catheter (Cardinal Health Monoject, Dublin, Ohio) was placed into a cephalic vein for collecting blood samples and administration of insulin and dextrose solution.
After measurements of baseline glucose concentration, regular human insulin (Humulin R, Eli Lilly and Company, Indianapolis, Indiana, 0.3 U/kg) was injected through the catheter. Blood was collected from the catheter at 10‐minute intervals for the rest of the 90 minutes. Two milliliters of contents was removed from the catheter (the dead space) and discarded using a syringe designated the “dead space syringe.” One milliliter of blood was then drawn from the catheter and 1 drop of the blood was used to measure glucose with the AlphaTRAK PBGM; the rest of the blood was collected in a lithium heparin blood tube and submitted within 60 minutes for analysis using a clinical chemistry analyzer (Vitros 4600, Ortho Diagnostics, Raritan, New Jersey) with BG reference interval: 60 to 135 mg/dL. An FGMS reader was waved over the subcutaneous glucose sensor to obtain a glucose reading from the FGMS at the same time as blood was drawn. At baseline, and again at 90 minutes, PCV was measured. Due to a miscommunication during data collection, PCV data were not available for 12 of the 24 dogs. To correct the hypoglycemia caused by administration of insulin, 50% dextrose solution (Vedco, Saint Joseph, Missouri) was diluted with sterile water to achieve 20% concentration and administered through the IV catheter. The replacement dose for dextrose was calculated for a hypothetical deficit of 60 mg/dL of extracellular fluid volume. The “60 mg/dL deficit” refers to a decrease in plasma glucose from 100 to 40 mg/dL. Estimated extracellular fluid volume was used for the calculation because glucose rapidly distributes into both the plasma and interstitial fluid when administered IV. A total of 100 mg/dL was used as “normal” BG concentration and 40 mg/dL was designated as “hypoglycemia needing correction.” Students administered the dextrose dose as a single bolus, not as a continuous infusion. A single 5 mg dose of dexamethasone (VetOne, Boise, Idaho) was administered SC. The median weight for the dogs was 24.5 kg, thus the 5 mg dose of dexamethasone resulted in a median dose of 0.2 mg/kg body weight, to achieve a 1‐time replacement of glucocorticoid activity dose. 14 These interventions were made at the discretion of each group of veterinary students under supervision of S.E. Washburn and C.A. Patterson. The timing and combinations of these interventions varied between dogs and was based on physical examination findings in conjunction with glucose concentrations. A complete physical examination was performed on each dog by a veterinarian (C.A. Patterson) prior to the laboratory. Students also performed a physical exam and monitored heart rate and mentation to detect hypoglycemia. They were required to measure BG concentration every 10 minutes from the time of insulin administration. Based on the heart rate and mentation of the dog, they could check the BG more frequently and potentially administer another correction (such as a dose of dextrose or food) if they observed changes (in mentation or heart rate) or overall trends in glucose concentrations. They did not have strict numerical criteria in regards to the heart rate; rather, they were instructed to track individual dog changes when making decisions. They were also instructed to administer a correction if BG concentrations were 40 mg/dL or below, regardless of any physical exam findings. The dogs were also fed a commercial dog food (Purina ProPlan puppy chicken & rice, St. Louis, Missouri) throughout the laboratory period at the discretion of the students. Once the BG concentration was stable, the catheter and sensor were removed and the dog was returned to its kennel.
2.3. Statistical analysis
Continuous data were assessed for normality using Anderson‐Darling tests and visual inspection QQ plots. Parametric data are presented as mean ± SD and nonparametric data are presented as median (min − max).
Glucose concentrations were compared between 2 test methods (PBGM and FGMS) and a reference method (clinical chemistry analyzer). Bland‐Altman plots were constructed and bias and 95% limits of agreement were calculated using a statistical software package (GraphPad Prism, version 5 for Windows, GraphPad Software, La Jolla, California).
Accuracy of the test methods was assessed according to ISO 15197:2013 criteria (BSI Standards Publication, in vitro diagnostic test system‐Requirements for BG monitoring system for self‐testing in managing DM; EN ISO 15197:2013) as described. 12 Briefly, mean absolute relative difference (MARD), median absolute relative difference (mARD), mean relative difference (MRD), and mean absolute difference (MAD) between the test methods (PBGM and FGMS) and the reference method were calculated to assess analytical accuracy. The number of pairs of glucose concentrations for which the test reading was within ±15 mg/dL of the reference BG reading for BG concentrations <100 mg/dL and within ±15% of the reference BG for reading ≥100 mg/dL were calculated. To be analytically accurate, ISO 15197:2013 criteria mandate that at least 95% of readings fall within these limits.
To assess clinical accuracy, Parkes consensus error grids for type 1 DM were plotted, whereby pairs of glucose readings are divided into 5 categories according to clinical risk (a‐e): (a) no effect on clinical action; (b) altered clinical action unlikely to affect outcome; (c) altered clinical action likely to affect clinical outcome; (d) altered clinical action could have substantial medical risk; and (e) altered clinical action could have dangerous consequence. 15 According to the ISO 15197:2013 criteria, 99% of the measured glucose results should fall within zones a and b. This analysis was performed with an open access statistical software package (getParkesZonesggplot and plotParkesGrid functions, ega [Error Grid Analysis] package [authors D Schmolze, S Mihhailov] for R studio, R Studio Team [2020]. RStudio: Integrated Development for R. RStudio, PBC, Boston, Massachusetts).
To further assess the accuracy of measuring IG after a rapid drop of BG concentration induced by exogenous insulin administration, changes in glucose concentration were described over time.
3. RESULTS
Twenty‐four recorded PCV measurements (at baseline and at 90 minutes for 12 of the 24 dogs), 23 (96%) were within the established reference interval used by the Texas A&M clinical pathology laboratory (37%‐56%). One reading (4%) was outside the lower bound of the reference interval at 27%. The mean ± SD baseline PCV was 40% ± 4% and the mean PCV at 90 minutes was 38% ± 5%.
Of a possible 240 glucose measurements, the reference method gave a reading 239 times and failed to give a reading on 1 occasion. One additional reference method result (581 mg/dL) was excluded from the analysis because it was severely discordant with both the PBGM result (36 mg/dL) and the FGMS result (67 mg/dL); it was assumed that the intravenous catheter had not been flushed adequately before sample collection. The median (min − max) concentration was 62 mg/dL (<20‐202 mg/dL). Out of a possible 240 readings, the PBGM gave a reading 239 times and failed to give a reading on 1 occasion (there was no reference method measurement for this time point either). There were 238 paired measurements for the reference method and the PBGM. The median (min − max) concentration was 72 mg/dL (27‐279 mg/dL). Out of a possible 240 readings, the FGMS gave a reading 223 times and failed to give a reading on 17 occasions. The reference method failed to give a reading once (at the aforementioned time point) and the previously described severely discordant result was excluded from analysis. Therefore, out of 240 possible readings, the FGMS failed to give a reading 17 times (17/240; sensor error rate of 7.1%) and 2 reference method readings were either not available or excluded; thus, there were paired 221 measurements for the reference method and the FGMS. The median (min − max) concentration was 79 mg/dL (<20‐139 mg/dL).
Bland‐Altman analysis of glucose measurements between the PBGM and clinical reference method showed a slight positive proportional bias and heteroscedasticity with more variation for higher glucose concentrations (Figure 1). Constant bias (95% limits of agreement) was estimated to be 8.4 mg/dL (−27.8 to 44.6 mg/dL). Bland‐Altman analysis of glucose measurements between the FGMS and clinical reference method showed considerable variation between methods and heteroscedasticity (greater variation for higher glucose concentrations; Figure 1). Constant bias was estimated to be 12.1 mg/dL and 95% limits of agreement were −39.3 to 63.6 mg/dL.
FIGURE 1.
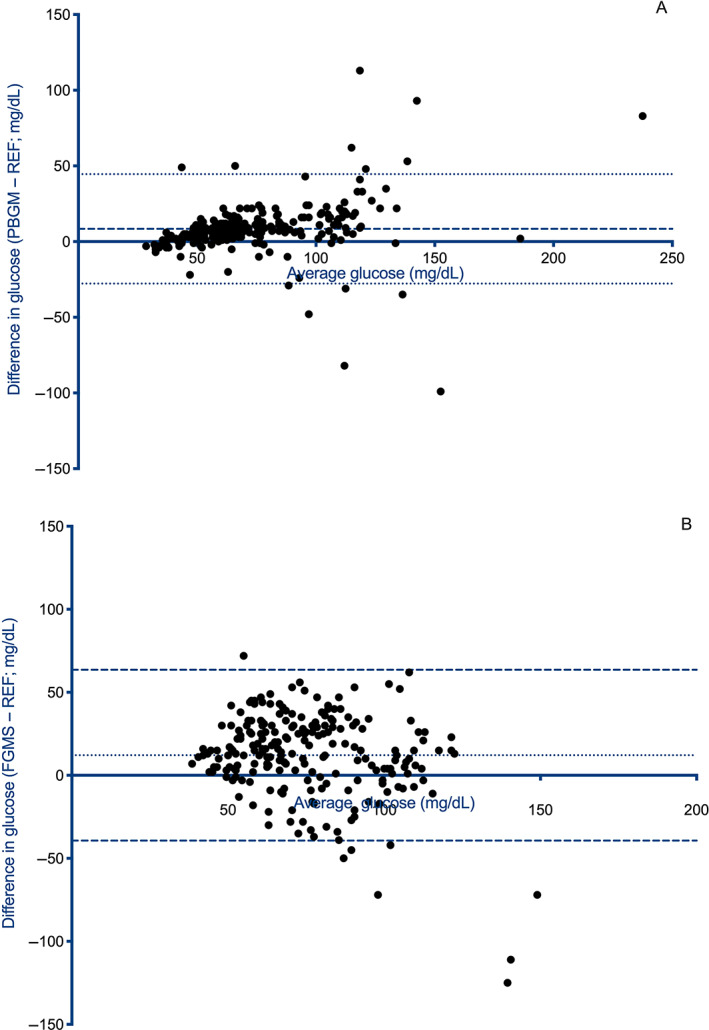
Bland‐Altman analysis of glucose measurements between (A) a portable blood glucose meter and (B) a flash glucose monitoring system and a reference method. (A) A slight positive proportional bias and heteroscedasticity with more variation for higher blood glucose concentrations is apparent. Constant bias was estimated to 8.4 mg/dL and 95% limits of agreement were −27.8 to 44.6 mg/dL. (B) Considerable variation between methods and heteroscedasticity (greater variation for higher glucose concentrations) are the most apparent findings. Constant bias was estimated to be 12.1 mg/dL and 95% limits of agreement were −39.3 to 63.6 mg/dL. FGMS, flash glucose monitoring system; PBGM, portable blood glucose meter; REF, reference standard
Mean absolute relative difference, MAD, mARD, and MRD between test methods and the reference method are presented in Table 1. The proportions of readings in the low BG range (reference method BG < 100 mg/dL) for which the test method measurement was within ±15 mg/dL of the reference BG for the PBGM and FGMS were 81.7% (161/197) and 39.1% (72/184), respectively; both of which are below the >95% mandated by ISO 15197:2013 criteria. The proportion of readings in the high BG range (reference method BG ≥ 100 mg/dL) within ±15% of the reference BG for the PBGM and FGMS were 44% (18/41)% and 54% (20/37), respectively; both of which are below the >95% mandated by ISO 15197:2013 criteria.
TABLE 1.
Analytical accuracy of a portable blood glucose meter and a flash glucose monitoring system
| Portable blood glucose meter | Flash glucose monitoring system | |
|---|---|---|
| Low range (reference method BG < 100 mg/dL) | ||
| n | 197 | 184 |
| MAD (mg/dL) | 10.6 | 22.3 |
| Percent of MAD values within ±15 mg/dL of the BG value | 81.7 (161/197) | 39.1 (72/184) |
| High range (reference method BG ≥ 100 mg/dL) | ||
| n | 41 | 37 |
| MARD (%) | 18.0 | 19.2 |
| mARD (%) | 17.3 | 13.6 |
| MRD (%) | 6.2 | −10.7 |
| Percent of values within ±15% of the BG value | 44 (18/41) | 54 (20/37) |
Note: The 2 test methods were compared to a reference standard. ISO 15197:2013 criteria mandate that at least 95% of readings fall within ±15 mg/dL of the reference BG reading for BG concentrations <100 mg/dL and within ±15% of the reference BG for reading ≥100 mg/dL.
Abbreviations: BG, blood glucose; MAD, mean absolute difference; MARD, mean absolute relative difference; mARD, median absolute relative difference; MRD, mean relative difference.
Parkes consensus error grids for the PBGM and FGMS are presented in Figure 2A,B. For the PBGM, 97.9% (233/238) of pairs were in zones a and b of the error grid. For the FGMS, 80.1% (177/221) of pairs were in zones a and b (Figure 2C). Based on the ISO 15197:2013 criteria, ≥99.0% of the measured glucose results should fall within zones a and b.
FIGURE 2.
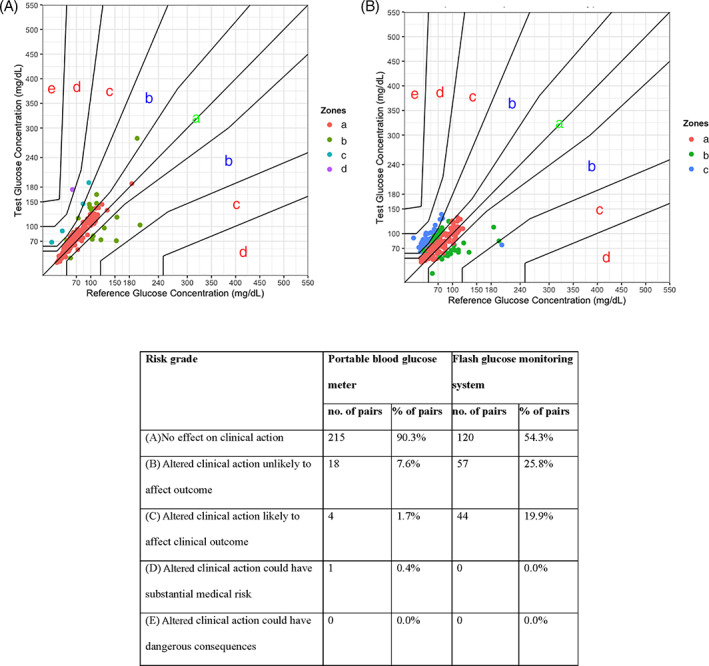
Parkes consensus error grid (for type 1 diabetes mellitus) of (A) a portable blood glucose monitor, (B) a flash glucose monitoring system, and (C) combined tabulated results. Zones are categorized as follows: (a) no effect on clinical action; (b) altered clinical action unlikely to affect outcome; (c) altered clinical action likely to affect clinical outcome; (d) altered clinical action could have substantial medical risk; and (e) altered clinical action could have dangerous consequence. Based on the ISO 15197:2013 criteria, ≥99% of the measured glucose results should fall within zones a and b
The changes in glucose concentration for the reference method and the PBGM were similar over time with the lowest median value for both at 20 minutes (Figure 3), followed by slight fluctuations. The glucose concentration for the FGMS was lowest at 50 minutes (Figure 3). There was considerable variation in glucose concentrations for each method between dogs as indicated by the wide interquartile ranges.
FIGURE 3.
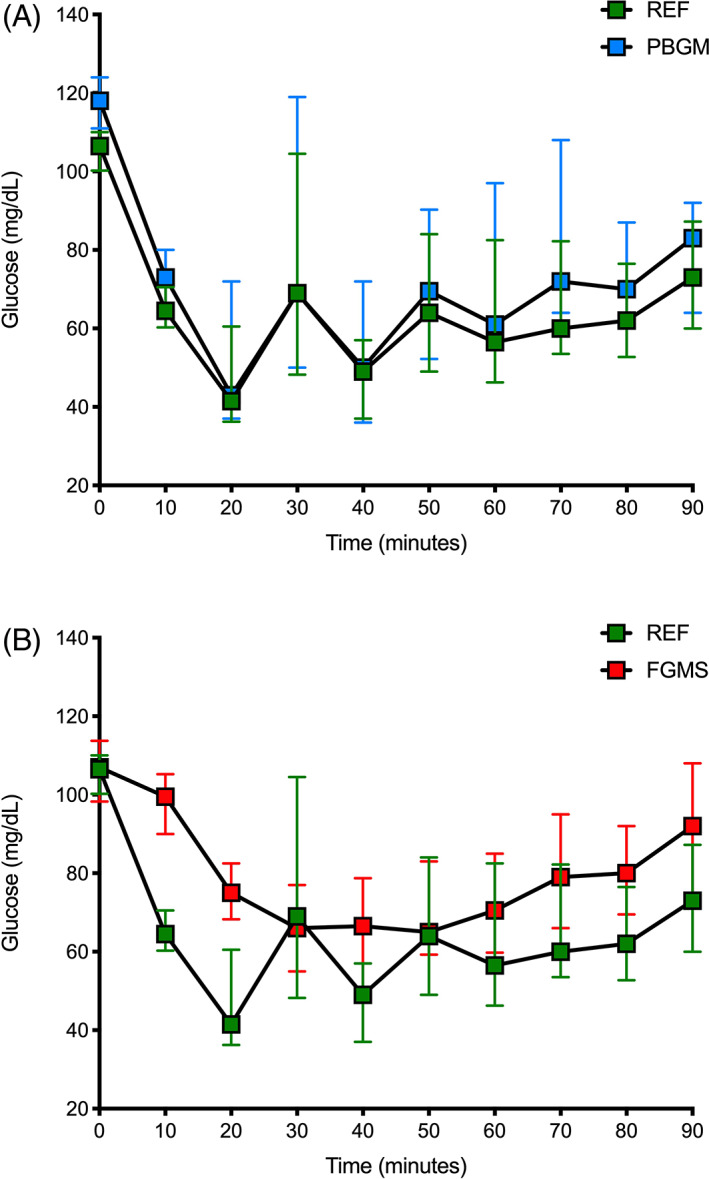
Changes in glucose over time for (A) portable blood glucose meter and the reference method and (B) flash glucose monitoring system (FGMS) and the reference method. Glucose concentrations in 24 dogs that were administered 0.3 U/kg regular insulin IV at time point 0 minute. Points and whiskers represent the median and interquartile range, respectively. (A) The lowest median glucose concentration for both methods occurred at 20 minutes; (B) The lowest median glucose concentration the reference method occurred at 20 minutes, whereas the lowest for the FGMS occurred at 50 minutes. PBGM, portable blood glucose meter; REF, reference standard
4. DISCUSSION
In this experimental model of healthy dogs with induced hypoglycemia and rapid fluctuations in BG concentrations, there was limited agreement between the FGMS and reference standard glucose measurements. Both the PBGM and the FGMS failed to meet ISO 15197:2013 criteria for analytical and clinical accuracy. The PBGM came close to meeting clinical accuracy requirements in this study, and clinical accuracy is demonstrated using error grid analysis (EGA) in other studies. 5 , 12 In dogs and cats with and without DM, and dogs with various diseases, this PBGM is clinically acceptable. 2 , 3 Earlier studies included a larger number of dogs than our study (155 dogs and 157 paired glucose measurements, respectively), and BG measurements varied across glycemic ranges (low, normal, and high glucose concentrations). The number of dogs and glycemic range data were more limited in our study. Because our study administered regular insulin to a study sample of nondiabetic dogs, inducing an acute change in BG, and rapidly reversing it with food, administration of intravenous dextrose, and a single dose of dexamethasone, it is difficult to directly compare results of our study to previous studies. The FGMS was not as accurate as compared to the PBGM and reference method, and failed to reliably detect hypoglycemia in our study. Our study did not assess the accuracy of the FGMS in hyperglycemic dogs. The correlation coefficients are r = 0.43, r = 0.50, and r = 0.85 for the hypoglycemic, normoglycemic, and hyperglycemic ranges, respectively. 5 However, our study looked at nondiabetic dogs with rapidly fluctuating BG concentrations, not diabetic dogs.
On Bland‐Altman analysis of the PBGM and FGMS, there was a positive bias for each in that on average the PBGM measured 8.4 mg/dL higher and the FGMS 12.1 mg/dL than REFERENCE. The 95% limits of agreement were wider for the FGMS than for the PBGM, which indicated the PBGM measurements were more closely related to REF, and thus more accurate in detecting BG concentrations under the specific conditions of this experimental model. There was greater variation from REF for higher glucose concentrations for both PBGM and FGMS.
The PBGM is most accurate, with ≤10 mg/dL deviation from the reference standard glucose concentration, when the subject's PCV is between 42% and 56% 16 ; however, our study was limited to investigating the change in PBGM readings in a blood sample which was subjected to hemoconcentration and hemodilution. Thus, extrapolations from existing data on the PBGM and PCV cannot be strictly applied to our study sample. Out of 24 PCV measurements, 8 were within the range 42% to 56%, 16 were below the range 42% to 56%, and none were above the range 42% to 56%. Although all but 1 PCV measurements were within the established reference interval used by the Texas A&M clinical pathology laboratory (37%‐56%), over half were considered hemodiluted based on the previous study of PBGM accuracy and PCV ranges. 16 The 1 PCV measurement below the reference interval (27% at 90 minutes) could be attributed to operator variability, whether in preparation or reading 17 ; alternatively, an extremely hemodiluted sample sent by the student group could have played a role. Hemodiluted samples allow a greater volume of plasma to react with the test strip reagent, resulting in higher measured glucose concentrations. 16 Measuring serum or plasma glucose concentrations with the PBGM has increased its accuracy. 18 PCV data were only available for half of the dogs in this study.
ISO 15197:2013 criteria are used in human medicine and are designed to evaluate methods of glucose measurement for accuracy in a manner that optimizes patient safety. 13 Smaller differences become increasingly important in the more dangerous hypoglycemic range (reference <100 g/dL). In contrast, there is a wider gap between glucose concentrations in the hyperglycemic range that impact clinical decision‐making. For the hypoglycemic category (reference method BG <100 mg/dL), only 39.1% (72/184) of the FGMS readings were within ±15 mg/dL of the reference BG measurement. The clinical relevance of the lack of agreement between the FGMS and the reference analyzer is illustrated by the Parkes error grid. This error grid was developed to evaluate the accuracy of BG measurements in self‐monitoring human patients with DM. 15 The risk categories were assigned by 100 American Diabetes Association physicians. In order for a device to be considered clinically accurate, at least 99% of the values have to fall within zones a and b, signifying no effect on clinical outcome. While the patient populations and clinical scenarios might be different, these guidelines for evaluating clinical accuracy are used as a framework for evaluating diagnostic performance of glucometers in veterinary medicine. 5 , 12 In this study, the percentage of FGMS readings in acceptable zones a and b was 80.1% (177/221), which means they were in close enough agreement that the dog's clinical outcome would not be affected. Alternatively, 19.9% (44/221) of readings would have led to an altered clinical action likely to affect dog outcome. However, the IG reference interval for normal, healthy dogs has not been established and this EGA relies on the BG intervals as a surrogate.
Similarly, the PBGM failed to meet ISO 15197:2013 criteria for clinical accuracy. For the hypoglycemic category (reference <100 mg/dL), 81.7% (161/197) of the PBGM readings were within ±15 mg/dL of the reference BG measurement. The percentage of readings that would not have affected clinical outcome was 97.9% (233/238). This is much closer to the 99% that would be deemed acceptable in human medicine. The PBGM was more analytically and clinically accurate than the FGMS in this sample of dogs. However, PBGM readings and outcomes were applied to a number, rather than any clinical signs suggestive of neuroglycopenia.
The FGMS was easy to apply and was well tolerated in all dogs included in the study. It is a practical and convenient tool that can provide more data and give a wider array of information about IG concentration trends over longer periods of time than is typically available for dogs. This FGMS is useful in the management of diabetic dogs, compared to BG curves using a PBGM, and the FGMS allows a more accurate assessment of glucose nadirs and day‐to‐day variations. 19 Use of the FGMS has become a popular choice in clinical practice, and its accuracy has been previously studied in outpatient diabetic dogs, as well as sick, hospitalized dogs with DKA. In outpatient diabetic dogs, clinical accuracy is 99% of results in zones a and b of the Parkes error grid analysis. 5 In dogs with DKA, clinical accuracy is >99% of results in zones a and b. 12 Additionally, the FGMS improves glucose control, as measured by reduced glycated hemoglobin Alc (HbA1c) in human diabetic patients, as well as limit glucose variability and reduce time during which patients are hypoglycemic. 20
The lack of reliability for the FGMS in our study is likely due, in part, to a lag in the IG concentration when BG changes quickly. Although the dynamic between blood and IG is complex and cannot simply be explained by a lag time, the equilibration between the 2 compartments when changes to BG occur is not instantaneous. Case studies in human diabetes show that considering the lag time does not allow for better reliability of IG monitoring. 11 The variable physiologic lag time is influenced by varying metabolic conditions, because glucose fluxes are largely insulin‐dependent. For example, a high plasma insulin concentration might result in hypoglycemia through various mechanisms affecting multiple physiologic compartments. 21 This scenario depicts a dynamic that is too complex to be accounted for by a simple calibration algorithm. This concept is important to keep in mind in situations when the BG changes quickly, such as after a dose of insulin is given. The same FGMS as in this study was used to compare glucose dynamics in the peritoneal cavity and the subcutaneous space of pigs and found that the intraperitoneal sensors responded faster to glucose excursions than did the subcutaneous sensors. 22 An FGMS traditionally placed in the subcutaneous space is much more convenient and might be reliable for monitoring trends over longer periods of time. The time it takes for the IG to equilibrate with the BG could lead to discrepancies that could affect clinical decision‐making and dog outcome. This physiologic lag time could actually be beneficial in some situations, as the IG might reflect a more stable and accurate estimation of the glucose available to cells in the body when BG is changing rapidly. When BG rather than IG is used as the “target” glucose for therapeutic decision‐making in humans, adverse patient outcomes can occur. 9 The FGMS measures glucose in the interstitial tissue fluid and provides an estimate of the BG concentration. None of the dogs in this study had observed neuroglycopenia or acute sympathoadrenal responses.
There were several limitations to this study, some of which are attributable to use of an induced model of hypoglycemia. Hyperglycemic readings were not obtained in this study. The study dogs received a variety of interventions at varying times after administration of insulin. The sampling method used in this study does not represent the repeated blood sampling that would occur for a diabetic dog hospitalized in a traditional intensive care unit setting. This study most closely mirrored a clinical scenario of insulin overdose; however, a dose of regular insulin was given IV as opposed to intermediate acting insulin given SC, which is the route used to administer insulin to diabetic patients and has a slower onset of effect. This sample of healthy teaching and research dogs might not be representative of the sample of dogs that are likely to use this FGMS (dogs with BG fluctuations). Several factors, such as hydration status, skin thickness, and BCS, affect the accuracy of IG monitoring. 23 The study sample was comprised of fairly uniform group of dogs and thus there was not important variation in BCS scores. All of these factors could be different between groups of healthy animals compared to dogs with an endocrinopathy or systemic illness. Whereas the sensor error rate in this study (7.1%; 17/240) was similar to those previously reported (10%‐25%, depending on sensor location 24 ), the number of sensor errors was a limitation, as these data points were lost. Additionally, a limitation to this study was its small sample size.
Our study demonstrated that, while beneficial in several other clinical situations, the FGMS is not reliable to detect hypoglycemia and rapid changes to BG concentration after administration of exogenous insulin to dogs. Whether the BG or IG is a more appropriate target glucose measurement for clinical decision‐making in this or other situations is unclear.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Approved by the Texas A&M University IACUC (#2019‐0224).
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
ACKNOWLEDGMENT
No funding was received for this study. The authors acknowledge Lisa Roberts‐Helton, Mandy Zachgo, Brianna Long, Mary Schacher, Stephanie Gamez, and the Texas A&M Doctor of Veterinary Medicine Class of 2023. The data from this study was presented as an abstract at the 2020 ACVIM Forum.
Howard LA, Lidbury JA, Jeffery N, Washburn SE, Patterson CA. Evaluation of a flash glucose monitoring system in nondiabetic dogs with rapidly changing blood glucose concentrations. J Vet Intern Med. 2021;35(6):2628-2635. doi: 10.1111/jvim.16273
REFERENCES
- 1. Cohen TA, Nelson RW, Kass PH, et al. Evaluation of six portable blood glucose meters for measuring blood glucose concentration in dogs. J Am Vet Med Assoc. 2009;235:276‐280. [DOI] [PubMed] [Google Scholar]
- 2. Kang MH, Kim DH, Jeong IS, et al. Evaluation of four portable blood glucose meters in diabetic and non‐diabetic dogs and cats. Vet Q. 2016;36:2‐9. [DOI] [PubMed] [Google Scholar]
- 3. Jahan S, Jamshidi S, Tehranisharif M, et al. Comparison of two veterinary blood glucose meters and one human‐based glucose meter for use in dogs. Iran J Vet Med. 2019;13:187‐198. [Google Scholar]
- 4. Abbott Laboratories . Free Style Libre 14 day system. https://www.freestyle.abbott/us‐en/support/faq.html?page=device/freestyle‐libre‐14‐day‐system/faq/topic/reader
- 5. Corradini S, Pilosio B, Dondi F, et al. Accuracy of a flash glucose monitoring system in diabetic dogs. J Vet Intern Med. 2016;30:983‐988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Surman S, Fleeman L. Continuous glucose monitoring in small animals. Vet Clin North Am Small Anim Pract. 2013;43:381‐406. [DOI] [PubMed] [Google Scholar]
- 7. Wiedmeyer CE, DeClue AE. Continuous glucose monitoring in dogs and cats. J Vet Intern Med. 2008;22:2‐8. [DOI] [PubMed] [Google Scholar]
- 8. Wiedmeyer CE, Johnson PJ, Cohn LA, et al. Evaluation of a continuous glucose monitoring system for use in veterinary medicine. Diabetes Technol Ther. 2005;7:885‐895. [DOI] [PubMed] [Google Scholar]
- 9. Siegmund T, Heinemann L, Kolassa R, et al. Discrepancies between blood glucose and interstitial glucose‐technological artifacts or physiology: implications for selection of the appropriate therapeutic target. J Diabetes Sci Technol. 2017;11:766‐772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rebrin K, Steil GM. Can interstitial glucose assessment replace blood glucose measurements? Diabetes Technol Ther. 2000;2:461‐472. [DOI] [PubMed] [Google Scholar]
- 11. Cobelli C, Schiavon M, Dalla Man C, et al. Interstitial fluid glucose is not just a shifted‐in‐time but a distorted mirror of blood glucose: insight from an in silico study. Diabetes Technol Ther. 2016;18:505‐511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Malerba E, Cattani C, Del Baldo F, et al. Accuracy of a flash glucose monitoring system in dogs with diabetic ketoacidosis. J Vet Intern Med. 2020;34:83‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. International Organization for Standardization . ISO 15197:2013: In Vitro Diagnostic Test Systems ‐ Requirements for Blood‐Glucose Monitoring Systems for Self‐Testing in Managing Diabetes Mellitus. Geneva, Switzerland: 2013: [Google Scholar]
- 14. Plumb DC. Dexamethasone. Plumb's Veterinary Drugs. In: Plumb DC, ed. Brief Media; 2015. ducational Concepts, LLC. dba Brief Media: Tulsa, Oklahoma. https://academic.plumbs.com/drug-monograph/uXRbPgQQYjPROD?searchQuery=dexam&source=search
- 15. Parkes JL, Slatin SL, Pardo S, et al. A new consensus error grid to evaluate the clinical significance of inaccuracies in the measurement of blood glucose. Diabetes Care. 2000;23:1143‐1148. [DOI] [PubMed] [Google Scholar]
- 16. Lane SL, Koenig A, Brainard BM. Formulation and validation of a predictive model to correct blood glucose concentrations obtained with a veterinary point‐of‐care glucometer in hemodiluted and hemoconcentrated canine blood samples. J Am Vet Med Assoc. 2015;246:307‐312. [DOI] [PubMed] [Google Scholar]
- 17. Breheny CR, Brown A, Handel I, et al. Inter‐ and intra‐operator variability in the analysis of packed cell volume. J Small Anim Pract. 2017;58:29‐34. [DOI] [PubMed] [Google Scholar]
- 18. Tauk BS, Drobatz KJ, Wallace KA, et al. Correlation between glucose concentrations in serum, plasma, and whole blood measured by a point‐of‐care glucometer and serum glucose concentration measured by an automated biochemical analyzer for canine and feline blood samples. J Am Vet Med Assoc. 2015;246:1327‐1333. [DOI] [PubMed] [Google Scholar]
- 19. Del Baldo F, Canton C, Testa S, et al. Comparison between a flash glucose monitoring system and a portable blood glucose meter for monitoring dogs with diabetes mellitus. J Vet Intern Med. 2020;34:2296‐2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Evans M, Welsh Z, Ells S, et al. The impact of flash glucose monitoring on glycaemic control as measured by HbA1c: a meta‐analysis of clinical trials and real‐world observational studies. Diabetes Ther. 2020;11:83‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rossetti P, Bondia J, Vehi J, et al. Estimating plasma glucose from interstitial glucose: the issue of calibration algorithms in commercial continuous glucose monitoring devices. Sensors. 2010;10:10936‐10952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Am MK, Kolle K, Fougner AL, et al. Effect of sensor location on continuous intraperitoneal glucose sensing in an animal model. PLoS One. 2018;13:e0205447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Reineke EL, Fletcher DJ, King LG, et al. Accuracy of a continuous glucose monitoring system in dogs and cats with diabetic ketoacidosis. J Vet Emerg Crit Care. 2010;20:303‐312. [DOI] [PubMed] [Google Scholar]
- 24. Hafner M, Lutz TA, Reusch CE, et al. Evaluation of sensor sites for continuous glucose monitoring in cats with diabetes mellitus. J Feline Med Surg. 2013;15:117‐123. [DOI] [PMC free article] [PubMed] [Google Scholar]


