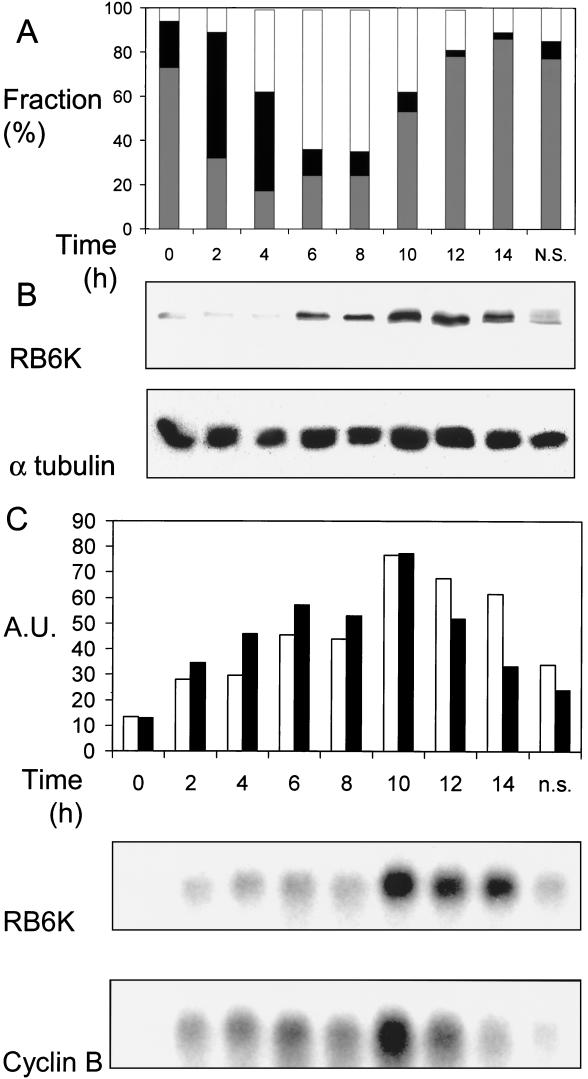
FIG. 3.
Expression of RB6K mRNA and protein in synchronized cultures. (A) Synchrony of cultures. Cells were synchronized as described in Materials and Methods. During progression through the cell cycle, cultures were harvested at 2-h intervals and the distribution of the cells over the stages of the cell cycle was determined by analyzing DNA contents using flow cytometry. The x axis shows time in hours after release from the hydroxyurea block. Bars: white, G2/M population; black, S population; grey, G1/G0 population. (B) RB6K protein expression. In parallel with the experiment shown in panel A, cell extracts were prepared and total protein was determined. Equal amounts were electrophoresed on an 8% (wt/vol) polyacrylamide gel under denaturing conditions and transferred to a Protran membrane, which was subsequently probed with anti-RB6K antibodies. As a control for equal loading the blot was reprobed with antibodies against α-tubulin. (C) RB6K mRNA levels and comparison to cyclin B. In parallel with the experiment shown in panel A, RNA was isolated. Ten micrograms of total RNA was denatured, electrophoresed, and blotted to Hybond-N filters. Filters were hybridized with radiolabeled probes for RB6K and cyclin B. After autoradiography and quantification, blots were stripped and reprobed for 28S RNA to allow normalization of the signals. The x axis shows time in hours after release from the hydroxyurea block. Bars: white, RB6K; black, cyclin B. n.s., nonsynchronized.