Figure 2.
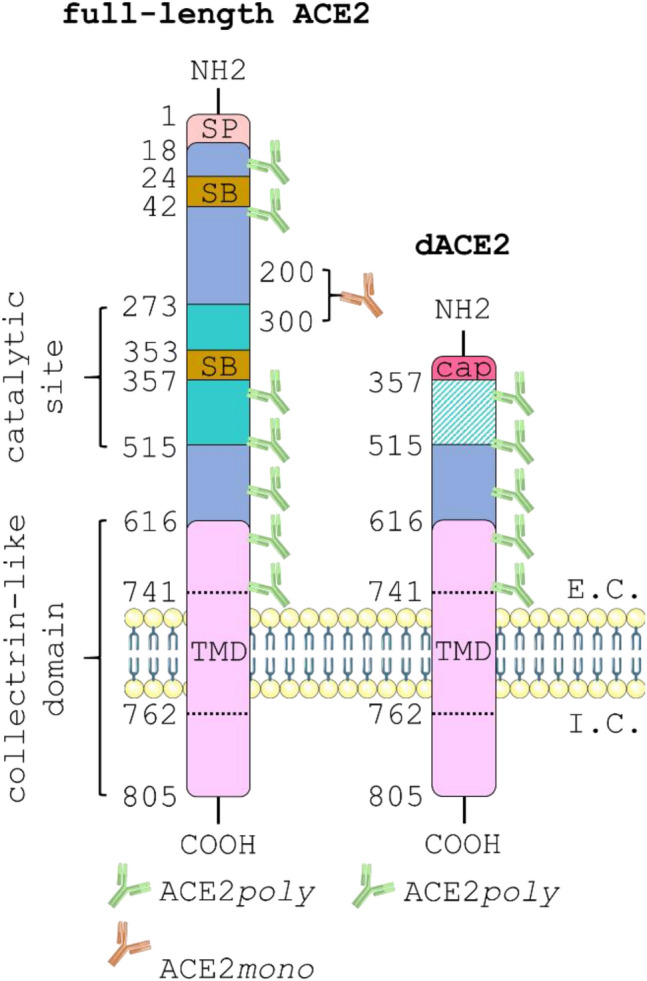
Schematic showing the critical protein domains of full-length ACE2 versus the short dACE2 isoform. The 805 amino acid full-length ACE2 protein (left) is comprised of an extracellular domain that protrudes into the extracellular (E.C.) space and an intracellular domain that remains in the intracellular (I.C.) space. The extracellular domain is made up of a signal peptide (SP) that extends from positions 1–18; the peptide-binding catalytic site that covers 272–515; two spike protein binding sites (SB) located at 24–42 and 353–357; a collectrin-like domain (CLD) that covers 616–805; and a short transmembrane domain (TMD) that spans the membrane at positions 741–762. The short dACE2 isoform (right) loses all amino acids up to positon 357 and a unique 10 amino acid sequence caps the N-terminus. Note that dACE2 has lost both its spike protein binding sites and the catalytic site is non-functional. The diagram also shows the potential binding sites for the ACE2poly antibody (green), raised against an 18–740 amino acid immunogen of ACE2, versus the single proprietary binding site that sits between amino acids 200–300 for the ACE2mono antibody (orange). If the full ACE2 isoform is present, green and orange fluorescent signal will be observed in immunological staining studies. If only the short ACE2 isoform is present, green fluorescent signal alone will be observed due to the lack of the monoclonal antibody binding site. The schematic was generated using templates from Servier Medical Art (smart.servier.com).
