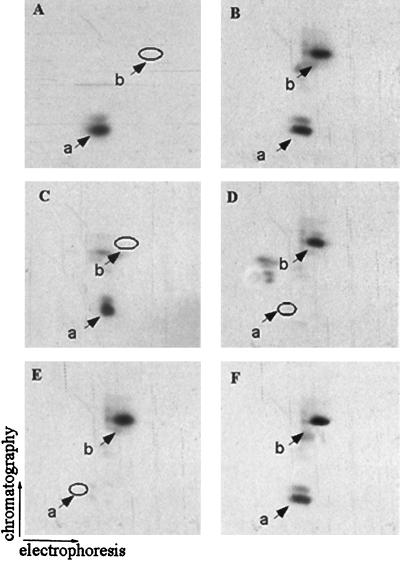
FIG. 5.
Characterization and purification of the tryptic peptide of PLC β1 phosphorylated by ERK. The 32P-labeled cells overexpressing PLC β1 were incubated without (A) or with (B) 40 ng of IGF-1/ml or with 50 ng of IGF-1/ml plus 50 μM PD98059 (C). Nuclear 32P-labeled PLC β1 was immunoprecipitated, separated by SDS–8% PAGE, and visualized by autoradiography as described for Fig. 1. The bands corresponding to PLC β1 were excised from the gels and digested in gel with trypsin. The tryptic peptide mixtures from each of these samples were analyzed by two-dimensional phosphopeptide mapping. (D) Tryptic peptides of PLC β1 phosphorylated in vitro by ERK. (E) HPLC fraction 36 as described in the text. (F) Mixture of peptides from panels B and E. The positions of phosphopeptides a and b are indicated.