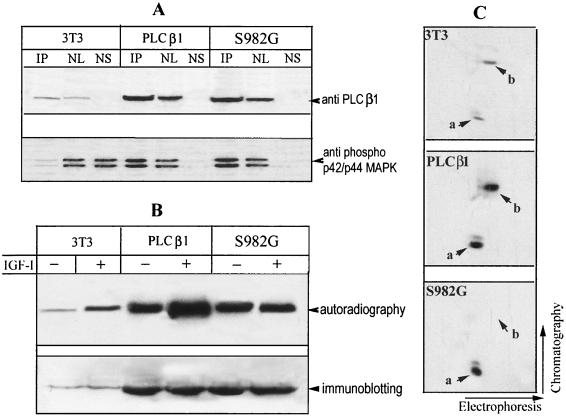
FIG. 9.
The PLC β1 S982G mutant associates with ERK and blocks ERK-mediated phosphorylation of endogenous PLC β1 in the nucleus. (A) Quiescent Swiss 3T3 cells and stable transfectants expressing wild-type PLC β1and S982G mutant cells (for details, see the legend to Fig. 7) were treated with 40 ng of IGF-I/ml for 5 min. The nuclei from these cells were purified and then lysed in IP buffer. Aliquots of nuclear lysate were subjected to IP by anti-PLC β1 MAb. IP complexes, nuclear lysate (NL), and nuclear supernatant after IP recovery (NS) were separated by SDS–8% PAGE and blotted with either anti-PLC β1 or anti-phospho-p42/p44 MAP kinase antibody, as described for Fig. 4. (B) Quiescent cells described for panel A were radiolabeled in vivo with [32P]orthophosphate for 4 h as in Fig. 2. Cells were then incubated without or with 40 ng of IGF-I/ml for another 5 min, and the nuclei from these cells were immunoprecipitated with anti-PLC β1 MAb. The immunocomplexes were separated by SDS-PAGE and then either analyzed by autoradiography or probed with anti-PLC β1 MAb, as for Fig. 2. (C) The 32P-labeled PLC β1 from IGF-I-treated 3T3 cells or cells overexpressing the S982G mutant was excised from the gels in panel B. The phosphoproteins were digested in gel by trypsin and analyzed by two-dimensional phosphopeptide mapping as described for Fig. 5. Note that phosphopeptide b was absent from cells expressing S982G. The figure is representative of three identical clones for each type of the transfectant.