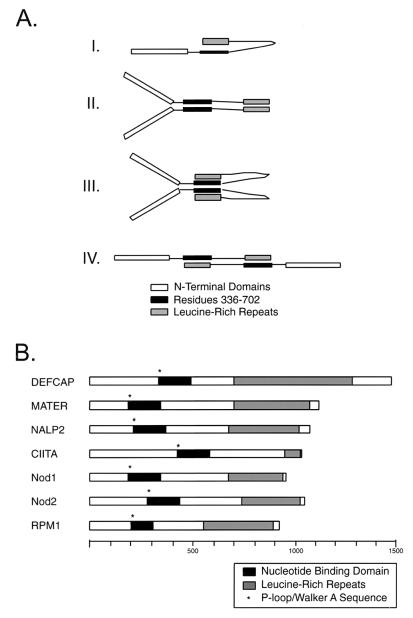
FIG. 7.
Model for self-association and similarity with other mammalian NBD-LRR proteins. (A) Graphical representation of the interactions described in this study. Conformation I would likely alternate with one of the other forms involving homotypic domain interactions (e.g., II or III). Conformation II is a dimeric configuration with homotypic domain interactions. This conformation would also likely alternate with another (e.g., I, III, or IV). Conformation III is a dimeric configuration with homotypic domain interaction (for amino acids 336 to 702) and heterotypic domain interaction (amino acids 336 to 702 with LRR). Multiple copies of this dimeric unit (4-mer, 6-mer, etc.) would allow for all observed interactions to occur simultaneously, obviating the need for additional (or transitional) states. Conformation IV is a dimeric configuration with heterotypic domain interactions. Again, alternating this configuration with other configurations (e.g., II or III) would be likely. (B) Schematic representation of recently described mammalian proteins with similarity to CIITA. Each protein contains a characterized or putative NBD and a series of C-terminal LRR sequences of varying number. NBD-LRR proteins are also common among plant disease resistance genes, as typified by RPM1. The scale shown indicates increments of 100 amino acids.