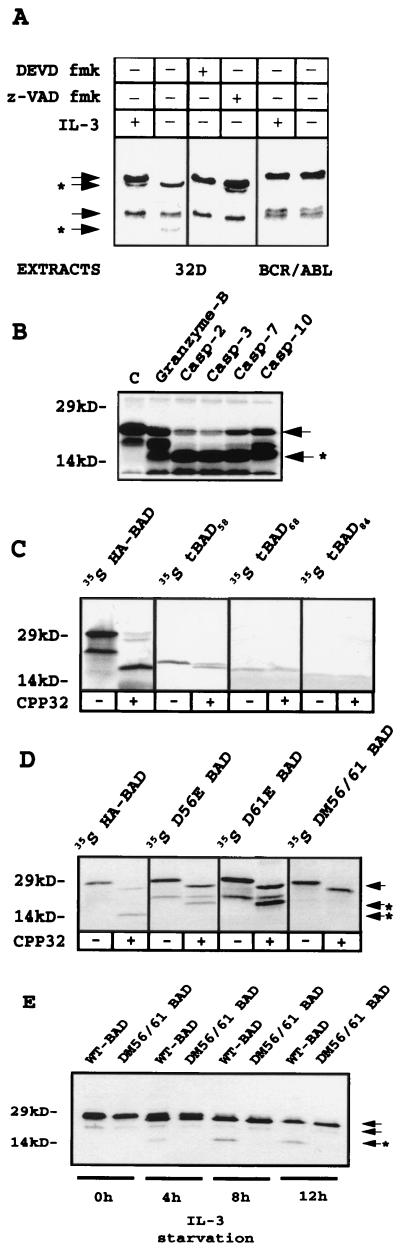
FIG. 2.
(A) Cleavage of in vitro-translated 35S-labeled murine HA-BAD by extracts of IL-3-starved (12 h) parental cells, untreated or treated with z-VAD fmk (50 μM) or DEVD fmk (50 nM), and nonstarved or starved (12 h) BCR/ABL-expressing 32Dcl3 cells. (B) Cleavage of in vitro-translated 35S-labeled murine BAD by recombinant granzyme-B, caspase 2, caspase 3, caspase 7, and caspase 10. Lane C contains the uncleaved in vitro translation products. (C and D) caspase 3-dependent cleavage of in vitro-translated 35S-labeled full-length, truncated, and mutant forms of murine HA-BAD. Reactions were carried out for 1 h at 30°C and stopped by adding sample buffer. The down-shift of full-length BAD produced by caspase 3 on WT and mutated BAD is probably due to the cleavage of the HA epitope, which contains a caspase 3 canonical cleavage site (DEVD). (E) Kinetics of WT and DM56/61 BAD-HA expression in virus-infected 32Dcl3 cells 0, 4, 8, and 12 h after IL-3 removal. Anti-BAD (C-20) antibody was used for Western blotting. Arrows indicate full-length BAD and a shorter translation product (∼23 kDa), presumably starting from methionine 43 of the full-length coding sequence; arrows plus asterisks indicate the cleavage products.