Highlights
-
•
CF bone disease is prevalent in children and adults despite recent advances in care.
-
•
DXA is the current gold standard in the evaluation of bone strength.
-
•
Limited evidence exists for the use of MRI or HRpQCT in the assessment of CFBD.
-
•
Fracture prediction models may help guide therapy but are not yet validated in CF.
Abbreviations: aBMD, areal bone mineral density; BMC, bone mineral content; BMD, bone mineral density; CF, Cystic Fibrosis; CFBD, Cystic fibrosis related bone disease; DXA, dual energy x-ray absorptiometry; FRAX, Fracture Risk Assessment Tool; HAZ, height for age Z-score; HSA, Hip Structural Analysis; ISCD, International Society Clinical Densitometry; LS, lumbar spine; LSC, least significant change; MRI, magnetic resonance imaging; pQCT, peripheral quantitative computed tomography; QUS, quantitative ultrasound; SOS, speed of sound; TBLH, total body less head; TBS, Trabecular Bone Score; UBPI, Ultrasound bone profile index; UD radius, Ultradistal radius; VFA, Vertebral Fracture Assessment
Keywords: Bone mineral density, Bone disease, Cystic fibrosis, Dual energy x-ray absorptiometry, Fracture
Abstract
With increasing life expectancy in people with Cystic fibrosis (CF), the focus of clinical care has shifted to management and prevention of non-pulmonary comorbidities. CF related bone disease, defined by low bone mineral density (BMD), is prevalent across all age groups and acknowledges the increased fractures rates that negatively impact lung function and quality of life. Dual energy X-ray absorptiometry (DXA) measurement of bone mineral content (BMC) and “areal” BMD (aBMD) is recommended for identifying and monitoring bone health in children and adults due to its low cost, low radiation exposure, and widespread availability. Recent studies in children and adolescents with chronic illness focus on adjustment of BMC and aBMD measurements for height due to the effects of short stature and delayed maturation on bone size. Expanded reference databases for alternate imaging sites such as the ultradistal radius and hip present opportunities for research and long-term monitoring. As the two-dimensional nature of DXA imposes limitations, we highlight other imaging modalities including peripheral quantitative computed tomography QCT (pQCT), magnetic resonance imaging, and quantitative ultrasound (QUS). These tools, while primarily used in a research setting, can impart information on true volumetric bone density and bone microarchitecture as well as contribute to fracture assessment and prediction. Due to the high morbidity and mortality associated with vertebral and hip fracture, we will present on vertebral fracture assessment (VFA) in both children and adults as well as applied analyses including hip structural analysis (HSA), trabecular bone score (TBS), and fracture risk assessment (FRAX) for high risk groups. Questions remain on the future clinical applicability and accessibility of these assessment and prediction tools, longitudinal monitoring through adolescence and adulthood, and how outcome measures may guide bone modifying therapies.
Background
The assessment and management of bone health in people with Cystic fibrosis (CF) presents an ongoing challenge despite advances in care over the past several decades. Improved survival amongst this population translates into a greater need for identifying and managing CF related bone disease (CFBD), defined by low bone mineral density (BMD) and increased risk of fragility fractures. The significance of CFBD is further underscored by its association with higher rates of morbidity and mortality and overall worse quality of life. The prevalence of low BMD has been reported in 20–38% of children and adults with CF [1], [2], [3], [4], [5] and increases with severity of lung disease, poor nutritional status, and age [6], [7], [8]. Risk factors for low bone mass accrual in CF include vitamin and mineral deficiencies, low body weight [3], [9], chronic inflammation and infection [10], impaired growth, reduced sex steroid hormones, CF related diabetes [11], and prolonged systemic glucocorticoid use [9], [10], [11], [12], [13]. Possible expression of the CF transmembrane conductance regulator (CFTR) in osteoblasts and osteoclasts may further contribute to reduced bone mineralization [8]. Multiple studies of bone histomorphometry and bone turnover in CF demonstrate accelerated bone resorption and low bone formation [2], [3], [4], [5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16] with resulting reduced trabecular bone volume and increased fracture [17], [18]. Vertebral and non-vertebral fractures are associated with worsening lung function, increased exacerbations and impaired airway clearance, worsening pain, and may be a contraindication to transplantation [1], [18], [19], [20]. While we continue to explore the multi-factorial basis of impaired bone quality and strength along with directed therapies to mitigate known risk factors, an understanding of how we assess bone health in children and adults through evaluation of density, geometry, and microarchitecture is critical to predicting and eventually reducing fracture risk. Research regarding bone health quantification relies largely on expert consensus, impacting our ability to establish consistent guidelines on bone health screening. This review will explore the current imaging tools of bone mineralization and their limitations in detection of low BMD in children and adults, as well as identify models of fracture assessment and prediction.
In 2019, the International Society of Clinical Densitometry (ISCD) published updated guidelines for low BMD and osteoporosis evaluation and highlighted the evolution of bone densitometry technology, reference databases, and reporting terminology [21]. “Low” BMD in post-menopausal females and males over 50 years of age is defined by BMD less than 1 standard deviation (SD) below the mean of healthy adults aged 30 years (T-score < -1.0 SD), while a T-score less than or equal to −2.5 SD or history of fragility fracture (fracture following a fall from standing height or vertebral fracture) is diagnostic of osteoporosis [21]. In young adults, adolescents, and children, bone density outcomes are adjusted for age, sex, and population specific normative data and low BMD is reported as Z-scores less than or equal to 2 standard deviations below the mean (Z-score ≤ − 2.0 SD) [21]. The diagnosis of osteoporosis in children and young adults cannot be made on BMD outcomes alone as reduced bone mineral content (BMC) or BMD Z-scores do not preclude the possibility of fragility fractures. In addition to Z-scores ≤ -2.0 SD, a history of clinically significant fractures (2 or more long bone fractures by 10 years of age or 3 or more long bone fractures by 19 years) must be present [21]. Low trauma vertebral fracture, with or without low BMD Z-scores, can also be diagnostic of osteoporosis. This more conservative definition reduces overdiagnosis and unnecessary treatment with bone modifying therapies. However, concerns have arisen that, particularly in high-risk youth, waiting for future fractures may lead to underdiagnosis and higher risk of osteoporosis [22].
Dual-energy X-ray absorptiometry
Dual-energy x-ray absorptiometry (DXA) is considered the gold standard for evaluation of BMC and BMD due to its widespread availability, low radiation dose exposure, low cost, ability to measure total body composition, and high degree of reproducibility [23]. DXA measures areal BMD (aBMD), the projection of BMC over imaged bone area (g/cm2) [24]. T-scores are generated as the difference in bone density from NHANES normative data, a reference population of healthy, young, white females [21] who are considered at peak bone mass and the lowest fracture risk [18]. Sites imaged in adults include the lumbar spine (LS, L1-L4) and the hip (including total hip and femoral neck) [Fig. 1]. The forearm (radius) may be included if the spine or hip cannot be imaged or if primary hyperparathyroidism is operative. Primary imaging sites in children and young adults include the LS and total body [Fig. 2]. aBMD and BMC for the total body is presented as the total body less head (TBLH) in order to subtract the mineral contribution of the larger cranium in young children. The non-linear nature of bone mineral accrual in childhood and adolescence necessitates reference data for comparison across age, sex, pubertal status, and ethnicity [24], [25]. The Bone Mineral Density in Childhood Study (BMDCS) is a multicenter national study with the purpose of creating sex and puberty specific normative aBMD and BMC data for children, adolescents, and young adults. While limitations in this dataset exist due to the study’s exclusion of children with severe short stature, delayed puberty, or chronic illness, expanded reference sets have been published for bone mineralization at the LS, TBLH, forearm, hip, femoral neck, and ultradistal (UD) radius [26], [27].
Fig. 1.
Representative lumbar spine (left) and hip (right) DXA.
Fig. 2.
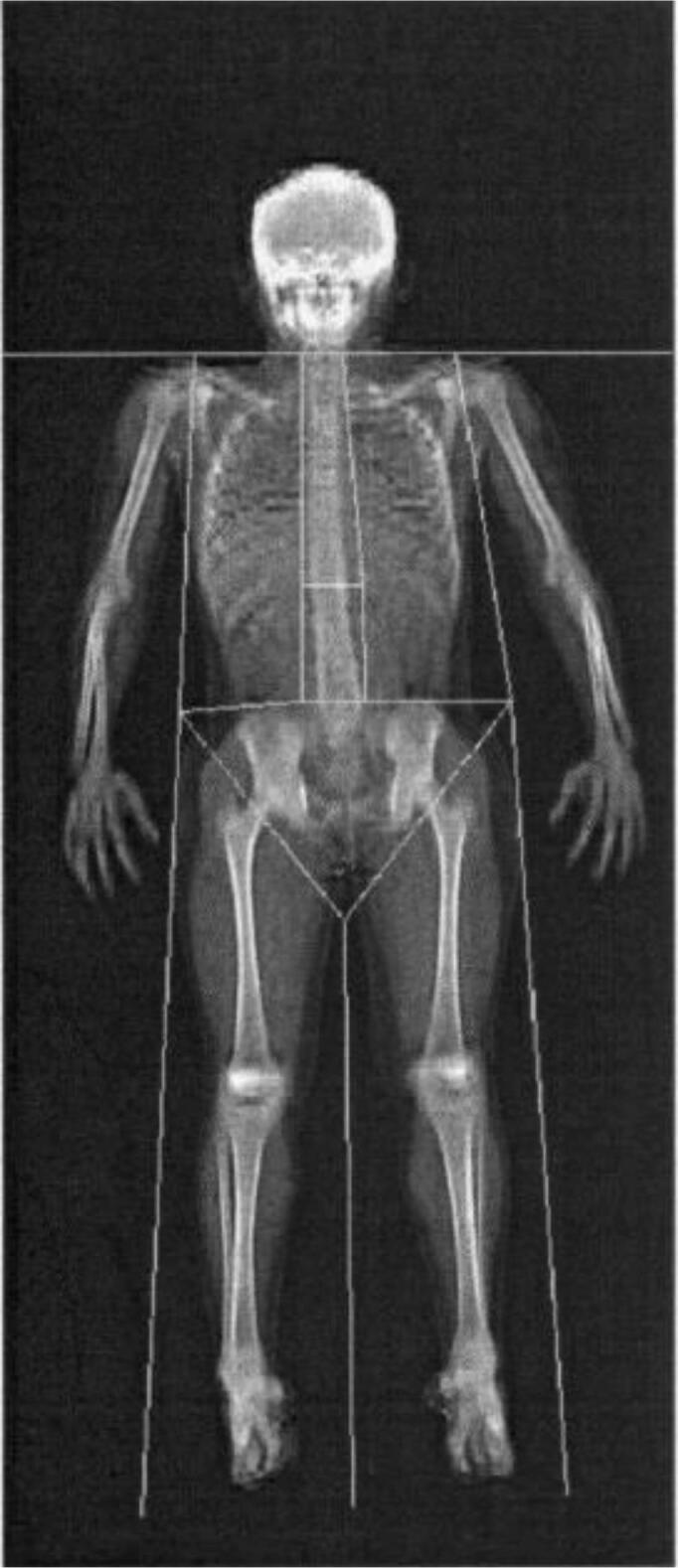
Representative total body DXA.
The radius has become a site of clinical interest in youth as the forearm is the most frequent fracture site in children and young adults [22]. This risk likely arises from rapid linear pubertal growth that outpaces BMC gains at the radius [28]. In CF, the effects of malnutrition and lower muscle mass further worsen radial bone strength [18]. DXA of the forearm includes two clinical sites: the distal 1/3 (33%) radius and the UD radius. Both locations have the advantage of being easily measured by DXA particularly in those who may have implanted hardware, significant scoliosis, or positioning difficulties that would impact the integrity of DXA imaging of the whole body or LS. UD radius aBMD Z-score outcome data correlate well with aBMD at the LS, total hip, and TBLH [27]. As it is rich in trabecular bone, the UD radius may help identify bone deficits in youth who are unable to undergo LS imaging [Fig. 3] However, at this time, the ISCD recommends using only the distal (1/3) radius for diagnostic purposes.
Fig. 3.
Representative forearm DXA (left). Location of the ultradistal (UD) radius and distal 1/3 radius in relation to the epiphyseal growth plate (A), including cross-sectional depictions of the high proportion of trabecular bone at the UD radius (B) and cortical bone at the distal 1/3 radius (right).
For patients with decreased ambulation, contractures, and risk of fragility fractures, the addition of bone health assessment at the hip and femur should be considered. DXA of the proximal hip includes the total hip, mostly comprised of cortical bone, and the trabecular rich femoral neck. Previously thought to be a less optimal imaging site in children and adolescents due to the variability in structural development during growth, the hip was traditionally not recommended for imaging; these concerns have been repudiated and normative data for BMC and aBMD are now available at these sites [24]. DXA of the lateral distal femur (LDF) includes three regions of interest: R1, the anterior distal metaphysis, R2, the metaphysis, and R3, the diaphysis. Studies have found BMC and aBMD at R1-R3 to correlate highly with measures of volumetric BMD [29] and a reliable alternate or additive site for bone density measurement. As per CF bone health guidelines, transition from monitoring DXA of the TBLH to the proximal femur should occur at age 20 [2]. Now that robust reference data are available, earlier monitoring of the hip in adolescents with CF may help with longitudinal tracking and guidance for initiation and monitoring of bone modifying therapeutic agents [30].
Bone mineralization T- and Z-scores are surrogate markers for fracture risk [24], [31], with studies reporting a two to three-fold increase in fracture probability for every 1 standard deviation decrease in aBMD [20]. In non-CF studies, BMC and aBMD Z-scores of the LS and TBLH correlate negatively with risk of vertebral and long bone fractures, respectively [24]. A decline in aBMD Z-score of greater than 0.5 is considered significant and for people treated with glucocorticoids, a greater decline in LS aBMD Z-score in the first 6 months of therapy is associated with increased incidence of vertebral fracture at 12 months [24]. Prospective studies evaluating DXA of the distal 1/3 and UD radius in healthy children correlate with increased probability of forearm fracture but are less predictive of long bone fractures in comparison to LS and total hip [30]. aBMD of the LDF has been reported as lower in non-ambulatory children with known history of lower extremity fracture [21], [30] but no longitudinal studies have examined subsequent fracture risk. Studies on hip aBMD and femoral fracture are limited due to the relatively low prevalence of hip fracture in youth. The relationship between fracture risk and BMD in CF remains conflicting. In a cross-sectional study of 43 patients with CF, Stahl et al found a negative association between aBMD Z-score and greater number of low trauma fractures [20]. Other cross-sectional studies have not shown BMD outcomes to be a predictor of bone fragility [23], [31]. No large-scale longitudinal studies assessing the magnitude of aBMD loss and fracture risk prediction have been conducted in CF. Overall, long term studies of change in bone density and fracture occurrence are needed in children and adults with CF.
Longitudinal monitoring of aBMD and BMC outcomes rely on the accuracy and precision of DXA machines and software as well as methods of quality control. The calculated result of precision assessment measurement, the precision error, represents the degree of reproducibility of BMC and BMD results. Precision relies on individual DXA instruments, the site of the body that is imaged, and the skill of the densitometry technician. The precision error is used to establish the least significant change (LSC), the smallest difference in serial DXA outcomes that can be considered statistically significant, and recognition of this parameter helps to guide initiation or monitoring of pharmacologic therapy. Regular quality control must be performed frequently at each clinical center. While the result is a high degree of reproducibility, the precision error and LSC are unique to each center and limit the ability to compare results across machine models. Thus, best practice is for patients to undergo follow-up imaging on the same DXA device [32].
Another limitation of DXA is the effect of growth and puberty on measures of bone mineralization. DXA calculates bone density using a 2-dimensional image of a 3-dimensional space, and is therefore at risk of incorrect estimations of bone depth and measures of true volumetric bone density. Unadjusted DXA results can underestimate bone mineral density for smaller size bones and overestimate for taller bones. Increased aBMD over time may be confounded by a positive change in bone size in growing children and a relative decline in Z-scores may be due to poor linear growth and smaller gains in mineralization. Earlier studies evaluating bone health in children and young adults with CF were notable for low bone density scores. In a 2008 study of 82 children and young adults with mild to moderate CF, TBLH and LS BMC Z-scores were found to be lower compared to healthy participants, however when adjusted for height Z-scores, the difference was no longer significant [33]. Incorporating height Z-score alone does not take into account the potential impact of delayed sexual maturation. Height for age Z-score (HAZ) adjustment of aBMD, accounting for variability in growth and puberty, has been found to be a better approach to minimize the effect of stature on mineralization [34]. In a recently published study of children with CF, baseline HAZ adjusted DXA Z-scores correlated strongly with future bone density [25], indicating that only certain patients may need more frequent bone health screening. Routine DXA reports will not include these adjustment results; however, online calculators are available to adjust aBMD and BMC for HAZ in children and young adults (https://zscore.research.chop.edu/calcpedbonedens.php) [35].
The ISCD also endorses another measure of height adjustment. Bone mineral apparent density (BMAD) uses a mathematical transformation of bone area to estimate the volumetric density and further approximate the effects of true bone size and depth. Age, sex, and population specific reference data are now available for LS BMAD for ages 5 years and older [36]. In comparison with other measures of aBMD, BMAD adjusted for HAZ best eliminates the effect of stature on bone density measurement [34] and is a strong predictor of vertebral fracture [37]. Clinicians should be aware that while bone size has an effect on measures of bone density and can underestimate bone density, bones of smaller size may not be as resistant to fracture as larger bones.
Other imaging
Although DXA is the currently recommended screening tool for bone health assessment, the issues of compromised accuracy due to short stature, requirement of specialized equipment, avoiding ionizing radiation exposure in patients who are pregnant, and inability to distinguish between cortical and trabecular bone have led to the need for other imaging modalities [38]. Additionally, utilizing alternate imaging tools can help identify early stages of altered microarchitecture and CFBD that may benefit from therapeutic interventions [39], [40]. As per the ISCD, alternative measures of bone density from other methodologies cannot be directly compared with each other or with DXA bone density measurements [21]. Thus far, none of the other imaging modalities have been shown to be superior to DXA in identifying increased fracture risk [2], [23], [41].
Quantitative ultrasound (QUS)
QUS is a low cost, portable method that calculates measures of bone mineral density, compressive strength, and elastic modulus (non-permanent resistance to deformation) without ionizing radiation. Measurements, including speed of sound (SOS, mg/cm3), the ultrasound bone profile index (UBPI), and amplitude-dependent speed of sound (Ad-Sos), are performed at peripheral sites, including the phalanges or calcaneus. In a study of 172 adults with CF, DXA-derived aBMD of the LS, proximal femur, and TBLH correlated strongly with calcaneal and phalangeal QUS measurements. However, only the UBPI, including phalangeal QUS measurements, was able to differentiate participants with and without vertebral fracture [42]. Mean T-score measures of SOS at the distal 1/3 radius in CF adults have been reported to be lower than healthy controls, with 14% of CF participants having SOS Z-scores in the lower end of normal range [39]. Other studies have not found differences between phalangeal Ad-SOS of children and young adults with CF compared to healthy age, sex, and BMI matched controls [40]. With regard to fracture prediction, QUS measurements at the calcaneus have been validated in postmenopausal women and men greater than 65 years and can be considered as a screening tool in patient populations with low likelihood of fracture [21].
Quantitative computed tomography (QCT) and peripheral QCT (pQCT)
QCT provides 3-dimensional volumetric BMD measurements (vBMD) and can differentiate between cortical and trabecular bone compartments [38]. pQCT and high-resolution pQCT (HR-pQCT) provide volumetric density measurements of the tibia and forearm and standard central QCT measure vBMD at the LS and hip. While HR-pQCT has the advantage of being less affected by bone size and surrounding adipose tissue, smaller trabecular and overall bone size with reduced muscle force and strain on bone may affect bone strength and pQCT outcome measures [38], [43]. QCT measures of vBMD have been studied in adolescents and young adults with CF and have identified abnormal bone microarchitecture, trabecular microstructure, cortical bone area, and estimated bone strength [44], [45], [46], [47] even when aBMD scores by DXA are reported as normal [48], [49], [50], indicating that pQCT may detect early bone deficits. The ISCD endorses the use of QCT for osteoporosis diagnosis in post-menopausal women and older men using femoral neck and total hip T-scores. In these patient populations, validated measures of vertebral fracture risk prediction by spine trabecular BMD and of hip fragility fractures from total femur trabecular and UD radius BMD measurements have been established. QCT, when combined with bone density scores from DXA, may have a high degree of sensitivity to estimate fracture prevalence and risk [20]. If LS or femoral DXA is not available for treatment decisions regarding therapeutic interventions, QCT derived density scores at the spine or pQCT density scores at the radius, taking into account clinical risk factors, may be used [21]. Disadvantages of QCT include higher cost and radiation exposure, therefore its use in patients with CF is limited to the research setting [18]. Larger prospective studies are needed to determine whether pQCT can identify young patients with CF that may be at increased risk of fracture not identified by DXA.
Magnetic resonance imaging (MRI)
In the clinical setting, MRI has been used to evaluate abnormal or equivocal reports of vertebral body anomalies and fracture obtained from both x-ray and DXA. MRI has theoretical advantages over DXA in its ability to evaluate bone microarchitecture, including distinguishing between cortical and trabecular bone, while avoiding radiation exposure. However, high cost and prolonged scanning times limit its use as a standard bone assessment tool [51]. The utility of MRI for screening, diagnosis, and monitoring for osteoporosis and fracture risk prediction has not been established and no studies of its use in patients with CF are available [18].
Fracture assessment and prediction methods
The ability of DXA, MRI, and HRpQCT to predict fracture in CF remains limited. Bone accrual in childhood is not linear and Z-score measures that are reported in range from one point in time do not preclude the risk of future fractures. The rate of change of bone density Z-scores may add to fracture prediction [52]; however, more data are needed in youth with chronic illness and reduced bone mineralization to confirm the utility of monitoring bone accrual. Due to the high morbidity and mortality associated with vertebral and hip fractures, other methods of fracture assessment and prediction should be considered when evaluating bone health but need to acknowledge that many of these methods are not yet validated in younger patients.
The risk of vertebral fracture increases with CF disease severity [53] and prolonged systemic glucocorticoid use [13]. Routine screening for children and adults that are considered high risk is necessary as vertebral fractures may be asymptomatic [23]. Vertebral Fracture Assessment (VFA) is a visual evaluation of the lateral projection of T4-L4 vertebrae via DXA imaging to detect vertebral body fracture. In comparison to traditional spine radiography, VFA is low cost, has lower radiation exposure, but is highly dependent on reader expertise as normal variation in vertebral body shape can be misinterpreted as a fracture [30]. Fracture identification utilizes the Genant semi-quantitative method [Fig. 4] which grades the type and severity of vertebral fracture by percent reduction in vertebral body heights [18], [24]. Indications to obtain VFA, if available, include low aBMD scores, history of prior vertebral fracture, and prolonged glucocorticoid use. In the research setting, VFA has been used to evaluate re-shaping and healing with and without bone modifying therapies [54]. VFA has been validated in children with osteoporosis [55] and leukemia [54], used in the longitudinal evaluation of vertebral fracture in adults with CF [53], and may aid in the prediction of future fracture [30], [54].
Fig. 4.
Adaptation of the Genant semi-quantitative method, the visual assessment of DXA-derived lumbar spine imaging to grade vertebral fracture by type (A - wedge fracture, B – biconcave fracture, and C – crush fracture) and severity, as defined by reduction in vertebral body height (grade I, mild, 20–24%, grade II, moderate 25–39%, and grade III, severe, greater than40%).
Bone disease can affect bone geometry and as a result, alter the biomechanical properties of bone and fracture risk [56]. Hip Structural Analysis (HSA) uses cross-sectional DXA data at the proximal femur to look at mineral mass distribution and hip strength in relation to femoral neck fracture risk. Cross-sectional area (CSA), cross-sectional moment of inertia (CSMI), and section modulus (Z), representing measures of bone surface area, bending stress, and bending strength, respectively, are calculated at 3 different locations of the femur [18]. CSA, CSMI, and Z correlate highly with measures of vBMD by pQCT [57]. Other measurements of hip geometry including hip axis length, femoral neck angle, and femoral neck width may have utility in fracture prediction [56], [58], [59]. However, they have not been studied in CF and reference data are not yet available for youth.
Trabecular bone score (TBS) yields complementary data to LS and femoral DXA using spatial models to provide information about bone microarchitecture. Lower TBSs correlate with weaker microarchitecture and increased risk of hip and vertebral fragility fractures in post-menopausal women and older men [21] and have been found to be an independent predictor of fracture risk with history of glucocorticoid use [18]. Insufficient data are available for TBS to be used as a fracture prediction tool or to initiate or monitor therapy in young people or people with CF. However, pediatric TBS reference data from the BMDCS are in preparation and may help inform utility of this measure in chronic health conditions like CF.
Lastly, FRAX (Fracture Risk Assessment Tool), an algorithm that calculates country-specific 10-year probability scores of hip and other fragility fractures, incorporates clinical risk factors such as age, weight, personal and family history of fracture, glucocorticoid use, and femoral neck aBMD [60]. FRAX can integrate TBS outcomes and can enhance fracture prediction when added to aBMD scores [21]. FRAX is only validated for people greater than 40 years of age and cannot be used to monitor the effects of treatment.
Conclusion
Current CF-specific guidelines recommend DXA as the primary imaging tool of bone health assessment with ongoing monitoring directed by age, pulmonary and nutritional status, glucocorticoid use, fracture history, and prior DXA outcome measures, if available. Evaluating DXA at the LS and hip in individuals aged ≥ 18 years and the LS and TBLH in youth remains the standard of care. Longitudinal studies evaluating bone health monitoring during the transition to adulthood will be critical to establish future screening guidelines, and expanding measures to include the hip in adolescence should be considered based on guidance from the ISCD now that robust reference data are now available for hip. The significant risk of morbidity and mortality accompanying vertebral fracture supports use of additional spine films or VFA, particularly in those with pain, low BMD scores, decreased height, or increasing vertebral body density. Further research is needed to validate the use of other imaging modalities, measures of bone microarchitecture, and fracture prediction tools in CF, particularly in children and young adults for whom reference data are lacking. Lastly, measures of bone density mineralization must be assessed in the setting of the clinical status trajectory of the individual so as to best guide ongoing treatment decisions and future health outcomes.
Funding sources
This work was supported by the Cystic Fibrosis Foundation EnVision-II CF: Emerging Leaders in CF Endocrinology [grant number WILLIA19GE0, DARUKH19GE0, HICKS19GE0, MORAN19GE3]. The sponsor had no role in study design, in the writing of the report, or in the decision to submit the article for publication.
CRediT authorship contribution statement
Kristen M. Williams: Conceptualization, Writing – original draft, Writing – review & editing, Visualization. Amy Darukhanavala: Writing – original draft, Writing – review & editing. Rebecca Hicks: Writing – original draft, Writing – review & editing. Andrea Kelly: Conceptualization, Writing – review & editing, Supervision.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We thank Dr. Andrew Aherne for his illustration contributions to this manuscript.
Contributor Information
Kristen M. Williams, Email: kmw2160@cumc.columbia.edu.
Amy Darukhanavala, Email: amy.darukhanavala@umassmemorial.org.
Rebecca Hicks, Email: rahicks@mednet.ucla.edu.
Andrea Kelly, Email: kellya@chop.edu.
References
- 1.Blackman S.M., Tangpricha V. Endocrine disorders in cystic fibrosis. Pediatr Clin North Am. 2016;63:699–708. doi: 10.1016/j.pcl.2015.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aris R.M., Merkel P.A., Bachrach L.K., Borowitz D.S., Boyle M.P., Elkin S.L., et al. Guide to bone health and disease in cystic fibrosis. J Clin Endocrinol Metab. 2005;90(3):1888–1896. doi: 10.1210/jc.2004-1629. [DOI] [PubMed] [Google Scholar]
- 3.Haworth C.S., Selby P.L., Webb A.K., Dodd M.E., Musson H., Niven R.M., et al. Low bone mineral density in adults with cystic fibrosis. Thorax. 1999;54(11):961–967. doi: 10.1136/thx.54.11.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bravo M.P., Balboa P., Torrejon C., Bozzo R., Boza M.L., Contreras I., et al. Bone mineral density, lung function, vitamin D and body composition in children and adolescents with cystic fibrosis: a multicenter study. Nutr Hosp. 2018;35:789–795. doi: 10.20960/nh.1609. [DOI] [PubMed] [Google Scholar]
- 5.Chirita-Emandi A, Shepherd S, Kyriakou A, McNeilly JD, Dryden C, Corrigan D, et al. A retrospective analysis of longitudinal changes in bone mineral content in cystic fibrosis. J Pediatr Endocrinol Metab 2017;30:807-14. https://doi.org/10.1515/jpem-2016-0057. [DOI] [PubMed]
- 6.Paccou J., Zeboulon N., Combescure C., Gossec L., Cortet B. The prevalence of osteoporosis, osteopenia, and fractures among adults with cystic fibrosis: a systematic literature review with meta-analysis. Calcif Tissue Int. 2010;86(1):1–7. doi: 10.1007/s00223-009-9316-9. [DOI] [PubMed] [Google Scholar]
- 7.Hendersen R.C., Madesen C.D. Bone density in children and adolescents with cystic fibrosis. J Pediatr. 1996;128:23–34. doi: 10.1016/s0022-3476(96)70424.9. [DOI] [PubMed] [Google Scholar]
- 8.Putman M.S., Baker J.F., Uluer A., Herlyn K., Lapey A., Sicilian L., et al. Trends in bone mineral density in young adults with cystic fibrosis over a 15 year period. J Cyst Fibros. 2015;14(4):526–532. doi: 10.1016/j.jcf.2015.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elkin S.L., Fairney A., Burnett S., Kemp M., Kyd P., Burgess J., et al. Vertebral deformities and low bone mineral density in adults with cystic fibrosis: a cross-sectional study. Osteoporos Int. 2001;12(5):366–372. doi: 10.1007/s001980170104. [DOI] [PubMed] [Google Scholar]
- 10.Ott S.M., Aitken M.L. Osteoporosis in patients with cystic fibrosis. Clin Chest Med. 1998;19(3):555–567. doi: 10.1016/s0272-5231(05)70100-3. [DOI] [PubMed] [Google Scholar]
- 11.Hardin D.S., Arumugam R., Seilheimer D.K., LeBlanc A., Ellis K.J. Normal bone mineral density in cystic fibrosis. Arch Dis Child. 2001;85:363–368. doi: 10.1136/adc.84.4.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Conway S.P., Morton A.M., Oldroyd B., Truscott J.G., White H., Smith A.H., et al. Osteoporosis and osteopenia in adults and adolescents with cystic fibrosis: prevalence and associated factors. Thorax. 2000;55:798–804. doi: 10.1136/thorax.55.9.798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weber D.R. Bone health in childhood chronic disease. Endocrinol Metab Clin North Am. 2020;49(4):637–650. doi: 10.1016/j.ecl.2020.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aris R.M., Ontjes D.A., Buell H.E., Blackwood A.D., Lark R.K., Caminiti M., et al. Abnormal bone turnover in cystic fibrosis adults. Osteoporos Int. 2002;13(2):151–157. doi: 10.1007/s001980200007. [DOI] [PubMed] [Google Scholar]
- 15.Gordon C.M., Binello E., LeBoff M.S., Wohl M.E., Rosen C.J., Colin A.A. Relationship between insulin-like growth factor 1, dehydroepiandrosterone sulfate and proresorptive cytokines and bone density in cystic fibrosis. Osteoporos Int. 2006;17:783–790. doi: 10.1007/s00198-005-0058-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grey A.B., Ames R.W., Matthews R.D., Reid I.R. Bone mineral density and body composition in adult patients with cystic fibrosis. Thorax. 1993;48(6):589–593. doi: 10.1136/thx.48.6.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elkin S.L., Vedi S., Bord S., Garrahan N.J., Hodson M.E., Compston J.E. Histomorphometric analysis of bone biopsies from the iliac crest of adults with cystic fibrosis. Am J Respir Crit Care Med. 2002;166(11):1470–1474. doi: 10.1164/rccm.200206-578OC. [DOI] [PubMed] [Google Scholar]
- 18.Anabtawi A., Trang L.e., Putman M., Tangpricha V., Bianchi M.L. Cystic fibrosis bone disease: pathophysiology, assessment and prognostic implications. J Cyst Fibros. 2019;18:S48–S55. doi: 10.1016/j.jcf.2019.08.018. [DOI] [PubMed] [Google Scholar]
- 19.Aris R.M., Renner J.B., Winders A.D., Buell H.E., Riggs D.B., Lester G.E., et al. Increased rate of fractures and severe kyphosis: sequelae of living into adulthood with cystic fibrosis. Ann Intern Med. 1998;128:186–193. doi: 10.7326/0003-4819-128-3-199802010-0004. [DOI] [PubMed] [Google Scholar]
- 20.Stahl M., Holfelder C., Kneppo C., Kieser M., Kasperk C., Schoenau E., et al. Multiple prevalent fractures in relation to macroscopic bone architecture in patients with cystic fibrosis. J Cyst Fibros. 2018;17(1):114–120. doi: 10.1016/j.jcf.2016.06.004. [DOI] [PubMed] [Google Scholar]
- 21.Shuhart C.R., Yeap S.S., Anderson P.A., Jankowski L.G., Lewiecki E.M., Morse L.R., et al. Executive summary of the 2019 ISCD position development conference on monitoring treatment, DXA cross-calibration and least significant change, spinal cord injury, periprosthetic and orthopedic bone health, transgender medicine, and pediatrics. J Clin Densit. 2019;22(4):453–471. doi: 10.1016/j.jocd.2019.07.001. [DOI] [PubMed] [Google Scholar]
- 22.Ward L.M., Weber D.R., Munns C.F., Hogler W., Zemel B.S. A contemporary view of the definition and diagnosis of osteoporosis in children and adolescents. J Clin Endocrinol Metab. 2020;105:e2088–e2097. doi: 10.1210/clinem/dgz294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sermet-Gadelus I., Bianchi M.L., Garabedian M., Aris R.M., Morton A., Hardin D.S. European cystic fibrosis bone mineralization guidelines. J Cyst Fibros. 2011;10:S16–S23. doi: 10.1016/S1569-1993(11)60004-0. [DOI] [PubMed] [Google Scholar]
- 24.Ward L.M., Konji V.N. Advances in the bone health assessment of children. Endocrinol Metab Clin North Am. 2020;49(4):613–636. doi: 10.1016/j.ecl.2020.07.005. [DOI] [PubMed] [Google Scholar]
- 25.Atlas G., Yap M., Lim A., Vidmar S., Smith N., King L., et al. The clinical features that contribute to poor bone heatlh in young Australians living with cystic fibrosis: A recommendation for BMD screening. Pediatr Pulmonol. 2021;56:2014–2022. doi: 10.1002/ppul.25375. [DOI] [PubMed] [Google Scholar]
- 26.Kalkwarf H.J., Zemel B.S., Gilsanz V., Lappe J.M., Horlick M., Oberfield S., et al. The bone mineral density in childhood study: bone mineral content and density according to age, sex, and race. J Clin Endocrinol Metab. 2007;92(6):2087–2099. doi: 10.1210/jc.2006-2553. [DOI] [PubMed] [Google Scholar]
- 27.Kindler JM, Kalkwarf H, Lappe JM, Gilsanz V, Oberfield SE, Shepherd JA, et al. Pediatric reference ranges for ultradistal radius bone density: results from the bone mineral density in childhood study. J Clin Endocrinol Metab 2020;105:e3529-39. https://doi.org/10.1210/clinem/dgaa380. [DOI] [PMC free article] [PubMed]
- 28.McCormack S.E., Cousminer D.L., Chesi A., Mitchell J.A., Roy S.M., Kalwarf H.J., et al. Association between linera growth and bone accrual in a diverse cohort of children and adolescents. JAMA Pediatr. 2017;171 doi: 10.1001/jamapediatrics.2017.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zemel B.S., Stallings V.A., Leonard M.B., Paulhamus D.R., Kecskemethy H.H., Harcke H.T., et al. Revised pediatric reference data for the lateral distal femur measured by Hologic Discovery/Delphi dual-energy x-ray absorptiometry. J Clin Densitom. 2009;12(2):207–218. doi: 10.1016/j.jocd.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weber D.R., Boyce A., Gordon C., Högler W., Kecskemethy H.H., Misra M., et al. ISCD Official Position. J Clin Densitom. 2019;22(4):567–589. doi: 10.1016/j.jocd.2019.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stephenson A., Jamal S., Dowdell T., Pearce D., Corey M., Tullis E. Prevalence of vertebral fractures in adults with cystic fibrosis and their relationship to bone mineral density. Chest. 2006;130(2):539–544. doi: 10.1378/chest.130.2.539. [DOI] [PubMed] [Google Scholar]
- 32.Zemel B.S., Wasserman H., Kelly A., Fan B.o., Shepherd J., Lappe J., et al. Intermachine differences in DXA measurements vary by skeletal site, and impact the assessment of low bone density in children. Bone. 2020;141:115581. doi: 10.1016/j.bone.2020.115581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kelly A., Schall J.I., Stallings V.A., Zemel B.S. Deficits in bone mineral content in children and adolescents with cystic fibrosis are related to height deficits. J Clin Densitom. 2008;11(4):581–589. doi: 10.1016/j.jocd.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zemel B.S., Leonard M.B., Kelly A., Lappe J.M., Gilsanz V., Oberfield S., et al. Height adjustment in assessing dual energy x-ray absorptiometry measurements of bone mass and density in children. J Clin Endocrinol Metab. 2010;95(3):1265–1273. doi: 10.1210/jc.2009-2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Children’s Hospital of Philadelphia Research Institute. Pediatric z-score calculator. https://zscore.research.chop.edu/calcpedbonedens.php. 2021 [accessed 1 Jul 2021].
- 36.Kindler J.M., Lappe J.M., Gilsanz V., Oberfield S., Shepherd J.A., Kelly A., et al. Lumbar spine bone mineral apparent density in children: results from the bone mineral density in childhood study. J Clin Endocrinol Metab. 2019 doi: 10.1210/jc.2018-01693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Crabtree N.J., Hogler W., Cooper M.S., Shaw N.J. Diagnostic evaluation fob one densitometric size adjustment techniques in children with and without low trauma fractures. Osteoporos Int. 2013;24:2015–2024. doi: 10.1007/s00198-012-2263-8. [DOI] [PubMed] [Google Scholar]
- 38.Brookes D.S.K., Briody J.N., Munns C.F., Davies P.S.W., Hill R.J. Cystic fibrosis-related bone disease in children: examination of peripheral quantitative computed tomography (pQCT) data. J Cyst Fibros. 2015;14(5):668–677. doi: 10.1016/j.jcf.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 39.Roggen I., Louis O., Van Biervliet S., Van Daele S., Robberecht E., De Wachter E., et al. Biervliet S, Van Daele S, Robberecht E, De Wachter E, et al Quantitative bone ultrasound at the distal radius in adults with cystic fibrosis. Ultrasound Med Biol. 2015;41(1):334–338. doi: 10.1016/j.ultrasmedbio.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 40.Lopez-Rodriguez M.J., Lavado-Garcia J.M., Canal-Macias M.L., Calderon-Garcia J.F., Moran J.M., Pedrera-Zamorano J.D. Quantitative ultrasound in Spanish children and young adults with cystic fibrosis. Bil Res Nurs. 2013;15(3):280–284. doi: 10.1177/1099800412441500. [DOI] [PubMed] [Google Scholar]
- 41.Sermet-Gaudelus I., Castanet M., Souberbielle J.C., Mallet E. Bone health in cystic fibrosis. Arch Pediatr. 2009;16:616–618. doi: 10.1016/s0929-693x(09)74088-6. [DOI] [PubMed] [Google Scholar]
- 42.Rossini M., Viapiana O., Del Marco A., de Terlizzi F., Gatti D., Adami S. Quantitative ultrasound in adults with cystic fibrosis: correlation with bone mineral density and risk of vertebral fractures. Calcif Tissue Int. 2007;80(1):44–49. doi: 10.1007/s00223-006-0117-0. [DOI] [PubMed] [Google Scholar]
- 43.Braun C., Bacchetta J., Braillon P., Chapurlat R., Drai J., Reix P. Children and adolescents with cystic fibrosis display moderate bone microarchitecture abnormalities: data from high-resolution peripheral quantitative computed tomography. Osteoporos Int. 2017;28(11):3179–3188. doi: 10.1007/s00198-017-4179-9. [DOI] [PubMed] [Google Scholar]
- 44.Putman M.S., Greenblatt L.B., Sicilian L., Uluer A., Lapey A., Sawicki G., et al. Young adults with cystic fibrosis have altered trabecular microstructure by ITS-based morphological analysis. Osteoporos Int. 2016;27(8):2497–2505. doi: 10.1007/s00198-016-3557-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Putman M.S., Milliren C.E., Derrico N., Uluer A., Sicilian L., Lapey A., et al. Compromised bone microarchitecture and estimated bone strength in young adults with cystic fibrosis. J Clin Endocrinol Metab. 2014;99(9):3399–3407. doi: 10.1210/jc.2014-1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nishiyama K.K., Agarwal S., Kepley A., Rosete F., Hu Y., Guo X.E., et al. Adults with cystic fibrosis have deficits in bone structure and strength at the distal tibia despite similar size and measuring standard and relative sites. Bone. 2018;107:181–187. doi: 10.1016/j.bone.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 47.Bai W., Binkley T.L., Wallace J.W., Carver T.W., Specker B.L. Peripheral quantitative computed tomography (pQCT) bone measurements in children with cystic fibrosis. Pediatr Pulmonol. 2016;51(1):28–33. doi: 10.1002/ppul.23323. [DOI] [PubMed] [Google Scholar]
- 48.O’Brien CE, Com G, Fowlkes J, Tang X, James LP. Peripheral quantitative computed tomography detects differences at the radius in prepubertal children with cystic fibrosis compared to healthy controls. PLoS One 2018;e0191013. https://doi.org/10.1371/journal.pone.019103. [DOI] [PMC free article] [PubMed]
- 49.Louis O., Clerinx P., Gies I., De Wachter E., De Schepper J. Well-nourished cystic fibrosis patients have normal mineral density, but reduced cortical thickness at the forearm. Osteoporos Int. 2009;20(2):309–314. doi: 10.1007/s00198-008-0646-7. [DOI] [PubMed] [Google Scholar]
- 50.Kelly A., Schall J., Stallings V.A., Zemel B.S. Trabecular and cortical bone deficits are present in children and adolescents with cystic fibrosis. Bone. 2016;90:7–14. doi: 10.1016/j.bone.2016.04.030. [DOI] [PubMed] [Google Scholar]
- 51.Chang G., Boone S., Martel D., Rajapakse C.S., Hallyburton R.S., Valko M., et al. MRI assessment of bone structure and microarchitecture. J Magn Reson Imaging. 2017;46(2):323–337. doi: 10.1002/jmri.25647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kelly A., Shults J., Mostoufi‐Moab S., McCormack S.E., Stallings V.A., Schall J.I., et al. Pediatric bone mineral accrual z-score calculation equations and their application in childhood disease. J Bone Miner Res. 2019;34(1):195–203. doi: 10.1002/jbmr.3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Papaioannou A., Kennedy C.C., Freitag A., O'Neill J., Pui M., Ioannidis G., et al. Longitudinal analysis of vertebral fracture and BMD in a Canadian cohort of adult cystic fibrosis patients. BMC Musculoskelet Disord. 2008;9(1) doi: 10.1186/1471-2474-9-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ward L.M., Ma J., Lang B., Ho J., Alos N., Matzinger M.A., et al. Bone morbidity and recovery in children with acute lymphoblastic leukemia: results of a six-year prospective cohort study. J Bone Miner Res. 2018;33(8):1435–1443. doi: 10.1002/jbmr.3447. [DOI] [PubMed] [Google Scholar]
- 55.Genant H.K., Engelke K., Fuerst T., Glüer C.-C., Grampp S., Harris S.T., et al. Noninvasive assessment of bone mineral and structure: state of the art. J Bone Miner Res. 1996;11(6):707–730. doi: 10.1002/jbmr.5650110602. [DOI] [PubMed] [Google Scholar]
- 56.Frysz M., Baird D., Gregory J.S., Aspden R.M., Lane N.E., Ohlsson C., et al. The influence of adult hip shape genetic variants on adolescent hip shape: findings from a population-based DXA study. Bone. 2021;143:115792. doi: 10.1016/j.bone.2020.115792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Joseph T.V., Caksa S., Misra M., Mitchell D.M. Hip structural analysis reveals impaired hip geometry in grils with type 1 diabetes. J Clin Endocrinol Metab. 2020;105:e4848–e4856. doi: 10.1210/clinem/dgaa647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fajar J.K., Taufan T., Syarif M., Azharuddin A. Hip geometry and femoral neck fractures: a meta-analysis. J Orthop Translat. 2018;13:1–6. doi: 10.1016/j.jot.2017.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Leslie W.D., Lix L.M., Morin S.N., Johansson H., Odén A., McCloskey E.V., et al. Hip axis length is a FRAX- and bone density-independent risk factor for hip fracture in women. J Clin Endocrinol Metab. 2015;100(5):2063–2070. doi: 10.1210/jc.2014-4390. [DOI] [PubMed] [Google Scholar]
- 60.FRAX Fracture Risk Assessment Tool, https://www.sheffield.ac.uk/FRAX/tool.aspx?country=9; 2011 [1 Jul 2021].





